
 ͭ���仯�����ڿ�ѧ�о���ҵ�����о���������;��
ͭ���仯�����ڿ�ѧ�о���ҵ�����о���������;�� ��
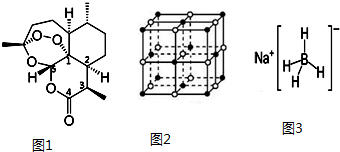
������ ��1����Cu��29��Ԫ�أ��۲���ӵ��Ų�Ϊ3d104s1���ݴ˻����۵��ӹ���Ų�ͼ��
�ڹ���е����Ų��ﵽȫ����������ȫ��ʱԭ�����ȶ���
��2�������������Һ����ȡԭ����д������ͭ�ܼ������ӷ���ʽ���ɣ�
��������У���������������֮������λ�����ڽ�����֮�������Ӽ��������Ӻ�ˮ�����ж��м��Թ��ۼ����ݴ˴��⣻
�۸������ӵ�����ԭ�Ӽ۲���Ӷ������µ��Ӷ������ж����ӵĿռ乹�ͣ����ݼ۲���ӶԻ�������ȷ��Sԭ�ӵ��ӻ���ʽ��
��3������Cu����ľ����ṹʾ��ͼ��֪���Զ���ͭԭ��Ϊ�������������ͭԭ��λ�ھ����������ϣ��ݴ˷���������$��=\frac{m}{V}$�����ܶȣ�
��� �⣺��1����Cu��29��Ԫ�أ��۲���ӵ��Ų�Ϊ3d104s1�����Լ۵��ӹ���Ų�ͼΪ ��
��
�ʴ�Ϊ�� ��
��
�ڹ���е����Ų��ﵽȫ����������ȫ��ʱԭ�����ȶ���Cu+�����������Ų�Ϊ3d10����Cu2+�����������Ų�Ϊ3d9�������������Ų��ﵽȫ��ʱ�ȶ������Թ�̬Cu2O�ȶ���ǿ��CuO��
�ʴ�Ϊ��Cu+�����������Ų�Ϊ3d10����Cu2+�����������Ų�Ϊ3d9�������������Ų��ﵽȫ��ʱ�ȶ������Թ�̬Cu2O�ȶ���ǿ��CuO��
��2����������ͭ�м��백ˮ���õ�����������ͭ���������ܽ�������ͭ��ԭ������ȡ�����Һ���ƣ��ʷ�Ӧ�����ӷ���ʽΪ��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��������У���������������֮������λ�����ڽ�����֮�������Ӽ��������Ӻ�ˮ�����ж��м��Թ��ۼ�������Cu��NH3��4SO4•H2O�����д��ڵĻ�ѧ�������Ӽ������Լ�����λ������ѡa��b��d��
��SO42-������ԭ����ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4��û�йµ��Ӷԣ�����SO42-�Ŀռ乹��Ϊ�������壬���ݼ۲���ӶԻ������ۿ�֪��Sԭ�ӵ��ӻ���ʽΪsp3��
�ʴ�Ϊ���������壻sp3��
��3������Cu����ľ����ṹʾ��ͼ��֪���Զ���ͭԭ��Ϊ�������������ͭԭ��λ�ھ����������ϣ�������ԭ����12�������Ծ�����Cuԭ�ӵ���λ��Ϊ12��Cu����ľ����к���ͭԭ����Ϊ$8��\frac{1}{8}+6��\frac{1}{2}$=4������$��=\frac{m}{V}$��֪�������ܶ�Ϊ$\frac{\frac{4��64}{{N}_{A}}}{��361.4��10{\;}^{-10}��{\;}^{3}}$g/cm3=$\frac{4��64}{[6.02��10{\;}^{23}����361.4��10{\;}^{-10}��{\;}^{3}]}$g/cm3��
�ʴ�Ϊ��12��$\frac{4��64}{[6.02��10{\;}^{23}����361.4��10{\;}^{-10}��{\;}^{3}]}$��
���� ���⿼�����ʽṹ�����ʣ���Щ֪ʶ�㶼��ѧϰ�ص㡢�߿��ȵ㣬�ѵ���ȷ�������ӻ�ѧʽ���Ѷ��еȣ������漰��������㣬Ӧע�⾧������������Ŀ��


 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | A��ǿ�ᡢBһ�������� | B�� | A�����ᡢBһ����ǿ�� | ||
| C�� | A��ǿ�ᡢB��ǿ�� | D�� | A����һ����B������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
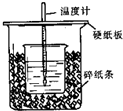 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
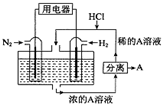 ��ѧ��������N2��H2Ϊ��Ӧ�������A��ϡ����Ϊ�������Һ��������������ṩ��Դ�����̵ܹ�������ȼ�ϵ�أ�װ����ͼ��ʾ������˵������ȷ���ǣ�������
��ѧ��������N2��H2Ϊ��Ӧ�������A��ϡ����Ϊ�������Һ��������������ṩ��Դ�����̵ܹ�������ȼ�ϵ�أ�װ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �õ�����·������ͨ��H2�ĵ�������ͨ��N2�ĵ缫 | |
| B�� | ��Ӧ��������Һ��pH������Ҫ�������� | |
| C�� | ͨ��N2�ĵ缫�����ĵ缫��ӦʽΪ��N2+6e-+8H+�T2NH4+ | |
| D�� | ͨ��H2�ĵ缫Ϊ������AΪNH4Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6.2 g | B�� | 8.8 g | C�� | 8.0 g | D�� | 11.2 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
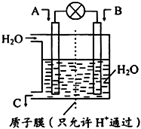 ȼú���ŷŴ�����CO��CO2��SO2��PM2.5������ο������ȾҲ������ȼú������أ�SO2��CO��CO2Ҳ�ǶԻ���Ӱ��ϴ�����壬�����ǵĺ������ơ��������Ż��������滷������Ч;����
ȼú���ŷŴ�����CO��CO2��SO2��PM2.5������ο������ȾҲ������ȼú������أ�SO2��CO��CO2Ҳ�ǶԻ���Ӱ��ϴ�����壬�����ǵĺ������ơ��������Ż��������滷������Ч;����| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������м�����ǿ����NaOH | B�� | ���ʻ�ԭ������ǿ������ | ||
| C�� | ���ʵ���ˮ��Ӧʱ������� | D�� | ԭ�Ӱ뾶������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com