



 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�ijС����ữ�����������������CO2������黯��Ӧ�������о���
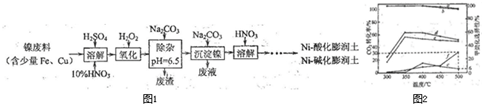
(1)�Ʊ�Niһ�ữ��������Ni-��������IJ����������£�
�١��ܽ⡱ʱά�ַ�Ӧ�¶�Ϊ70~80�棬��Ŀ���� ��
�ڡ�������һ����Ӧ�����ӷ���ʽΪ ��
��2�����һ����HNO3��ɻ��յ���Ҫ���ʣ�д��ѧʽ�� ��
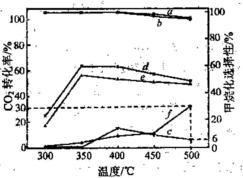
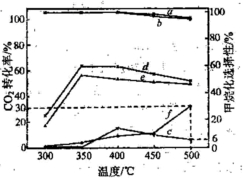
(3)�ڲ�ͬ�¶��£���������Ni-�ữ��������Ni-���������CO2������黯��Ӧ�������ͼ��ʾ��
�� �ڲⶨ�¶��ڣ�Ni-�ữ��������CO2������黯��Ӧ���������¶�Ϊ350�棬�����ǣ� �������������������������������������������������� ��
�� 500��ʱ��������ʵ����������ijװ�����������ܱ�������ͨ��5 mol CO2��20 mol H2����ַ�Ӧ������CH4�����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��У������ѧ�ڵ�����ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��16�֣�ijС����ữ�����������������CO2������黯��Ӧ�������о���
(1)�Ʊ�Niһ�ữ��������Ni-��������IJ����������£�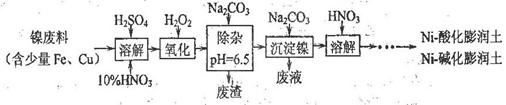
�١��ܽ⡱ʱά�ַ�Ӧ�¶�Ϊ70~80�棬��Ŀ���� ��
�ڡ�������һ����Ӧ�����ӷ���ʽΪ ��
��2�����һ����HNO3��ɻ��յ���Ҫ���ʣ�д��ѧʽ�� ��
(3)�ڲ�ͬ�¶��£���������Ni-�ữ��������Ni-���������CO2������黯��Ӧ�������ͼ��ʾ��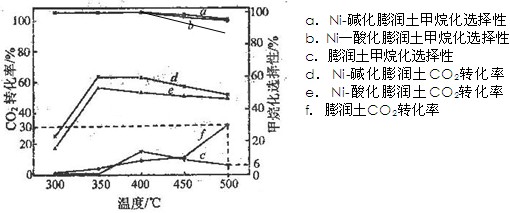
�� �ڲⶨ�¶��ڣ�Ni-�ữ��������CO2������黯��Ӧ���������¶�Ϊ350�棬�����ǣ� �������������������������������������������������� ��
�� 500��ʱ��������ʵ����������ijװ�����������ܱ�������ͨ��5 mol CO2��20 mol H2����ַ�Ӧ������CH4�����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��У������ѧ�ڵ�����ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��16�֣�ijС����ữ�����������������CO2������黯��Ӧ�������о���
(1)�Ʊ�Niһ�ữ��������Ni-��������IJ����������£�
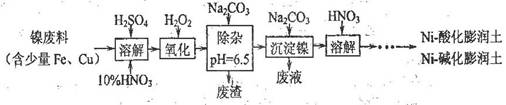
�١��ܽ⡱ʱά�ַ�Ӧ�¶�Ϊ70~80�棬��Ŀ���� ��
�ڡ�������һ����Ӧ�����ӷ���ʽΪ ��
��2�����һ����HNO3��ɻ��յ���Ҫ���ʣ�д��ѧʽ�� ��
(3)�ڲ�ͬ�¶��£���������Ni-�ữ��������Ni-���������CO2������黯��Ӧ�������ͼ��ʾ��
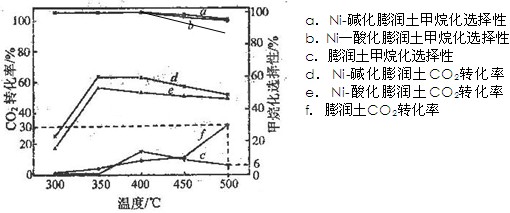
�� �ڲⶨ�¶��ڣ�Ni-�ữ��������CO2������黯��Ӧ���������¶�Ϊ350�棬�����ǣ� �������������������������������������������������� ��
�� 500��ʱ��������ʵ����������ijװ�����������ܱ�������ͨ��5 mol CO2��20 mol H2����ַ�Ӧ������CH4�����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ�Ͼ��и߿���ѧ��ģ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com