
����Ŀ��һ��Ru�������g-C3N4���Ϲ������CO����ԭΪHCOOH��ԭ��ͼ��ͼ��

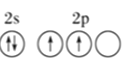
(1)��̬̼ԭ�ӵļ۵����Ų�ͼΪ___________��
(2)1molHCOOH�к��е�������ĿΪ___________��HCOOH�ķе��CO2�ߵ�ԭ��Ϊ___________��
(3)Ru������еڶ�����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��
(4)Ru���������Ru��λ��ԭ����N��___________��
(5)һ��ʯī�ľۺ���뵼��g-C3N4���䵥��ƽ��ṹ��ͼ1�������ṹ��ͼ2��
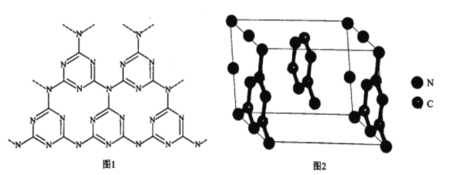
��g-C3N4�е�ԭ�ӵ��ӻ�������______________��
�ڸ���ͼ2����ͼ1����ƽ���ı��λ���һ����С�ظ���Ԫ��______________
����֪�þ��������ΪVcm3���м��ԭ�Ӿ��ھ����ڲ����谢���ӵ�������ֵΪNA����g-C3N4���ܶ�Ϊ______________g.cm-3��
���𰸡�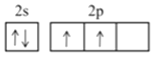 ��
�� 4NA HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ���� N>O>C Cl��C sp2�ӻ�
4NA HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ���� N>O>C Cl��C sp2�ӻ� 
![]()
��������
�����������ԭ�����ɻ�̬̼ԭ�ӵļ۵����Ų�ʽȷ����۵����Ų�ͼ��HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ�����������·е�ƫ�ߣ�ͬһ����Ԫ�صĵ�һ����������ԭ�������������������ͬһ���ڵĵڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ��ڢ�A��Ĵ��ڵڢ�A��ģ���ͼһ��֪N�γ�������ѧ������3���ӻ�������ӻ������ļн�Ϊ120����
(1)�����������ԭ������̬̼ԭ�ӵļ۵����Ų�ʽΪ2s22p2������۵����Ų�ͼΪ![]() ��
��![]() ��
��
(2)һ����������һ������,һ��˫���к���һ������,һ�����������ݼ�����ӽṹ��֪1���Ӽ����к�4����������1molHCOOH�к��е�������ĿΪ4NA��HCOOH��CO2��Ϊ���Ӿ��壬��HCOOH���Ӽ��γ���������������ͨ���Ӽ�������Ҫǿ����HCOOH�ķе��CO2�ߣ�
(3)Ru������к��еĵڶ�����Ԫ��ΪC��N��O��ͬһ����Ԫ�صĵ�һ����������ԭ�������������������ͬһ���ڵĵڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ��ڢ�A��Ĵ��ڵڢ�A��ģ��ʵ�һ�������ɴ�С��˳��ΪN>O>C��
(4)��Ru�����Ľṹ��֪��������Ru��λ��ԭ����N��Cl��C��
(5)����ͼһ��֪N�γ�������ѧ������3���ӻ�������ӻ������ļн�Ϊ120������Nԭ�ӵ��ӻ�����Ϊsp2�ӻ���
�ڸ���ͼ2����ͼ1����ƽ���ı��λ���һ����С�ظ���ԪΪ ��
��
����=m/V=M/VNA=![]() g.cm-3
g.cm-3


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��Ǹ������������������Ϊ�����һ����¯��������Ϊ�ձ������������
I.��֪��Ӧ![]() Fe2O3(s)+CO(g)
Fe2O3(s)+CO(g)![]() Fe(s)+CO2(g)��H=-23.5kJmol-1���÷�Ӧ��1000����ƽ�ⳣ������4����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol��Ӧ����l0min��ﵽƽ�⡣
Fe(s)+CO2(g)��H=-23.5kJmol-1���÷�Ӧ��1000����ƽ�ⳣ������4����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol��Ӧ����l0min��ﵽƽ�⡣
(1)CO��ƽ��ת����=__��
(2)�����CO��ƽ��ת���ʣ��ٽ�Fe2O3��ת�����ɲ�ȡ�Ĵ�ʩ��__��
a.��߷�Ӧ�¶�
b.����Ӧ��ϵ��ѹǿ
c.ѡȡ���ʵĴ���
d.��ʱ���ջ��Ƴ�����CO2
e.�����ʯ��ʹ����ƽ���������ֽӴ�
��.��¯���������ķ����е�CO�ɽ��л��գ�ʹ����һ�������º�H2��Ӧ�Ʊ��״���CO(g)+2H2(g)![]() CH3OH(g)�������ͼʾ�ش��������⣺
CH3OH(g)�������ͼʾ�ش��������⣺
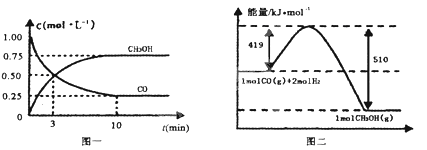
(3)�ӷ�Ӧ��ʼ��ƽ�⣬��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(H2)=___��
(4)��֪������ȼ����286kJ/mol����д���״����岻���ȼ�յ��Ȼ�ѧ����ʽ___��
(5)�����¶Ⱥ�������ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ�ⅼ���й��������±���
���� | ��Ӧ��Ͷ����� | ��Ӧ��� ת���� | CH3OH ��Ũ�� | �����仯(Q1��Q2��Q3������0) |
�� | 1molCO��2molH2 | ��1 | c1 | �ų�Q1kJ���� |
�� | 1molCH3OH | ��2 | c2 | ����Q2kJ���� |
�� | 2molCO��4molH2 | ��3 | c3 | �ų�Q3kJ���� |
�����й�ϵ��ȷ����___��
A.c1=c2 B.2Q1=Q3 C.2��1=��3 D.��/span>1+��2=1
E.�÷�Ӧ������1molCH3OH����ų�(Q1+Q2)kJ����
��.�Լ���Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺
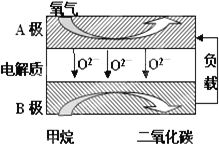
(6)B���ϵĵ缫��ӦʽΪ___��
(7)���ø�ȼ�ϵ������Դ����ʯī���缫���100mL1mol/L������ͭ��Һ���������ռ���������������ʱ�����������ĵļ�������Ϊ__(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ�ζ�������ͼ��ʾ������˵����ȷ����
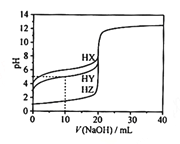
A.����ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX
B.���ݵζ����ߣ��ɵ�Ka(HY)��10��6
C.������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(Na+)��c(H��)
D.HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)��c(Y��)��c(Z��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������X��Y��Z����Է���������ϵΪMr(X)<Mr(Y)��0.5Mr(Z)������˵����ȷ����
A.ԭ����Ŀ��ȵ��������壬����������Z
B.ͬ��ͬѹ�£�ͬ�������������壬�����ܶ���С����X
C.ͬ��ͬѹ�£��������������Ϊ6.72 L�������ǵ����ʵ���һ����Ϊ0.3 mol
D.ͬ���£������ͬ���������ֱ����2 g Y�����1 g Z���壬����ѹǿ��Ϊ2�U1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
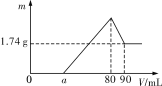
����Ŀ��һ��������þ���������Ͷ��2mol��L��1�������У���������ȫ�ܽ��������Һ����μ���2mol��L��1������������Һ�����ɳ������������������������Һ�������ϵ��ͼ��ʾ��
��
��1��0��amL��a��80mL��80��90mL��Ӧ��Ӧ�����ӷ���ʽ�ֱ�Ϊ��
��0��amL��___��
��amL��80mL��__��__��
��80mL��90ml��___��
��2��������������Ϊ___��
��3������������__mL��
��4��![]() =__��
=__��
��5��a=__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
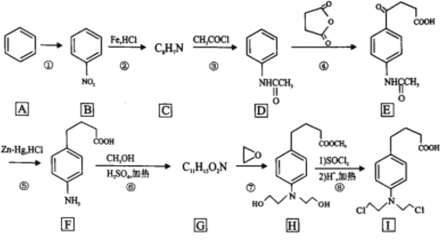
����Ŀ�������ᵪ���ǵ������ҩ�Ĵ������ϳ�·����ͼ��ʾ��
�ش��������⣺
(1)��Ӧ��������Լ���������______________��B�еĹ�����������______________
(2)C�Ľṹ��ʽΪ______________��
(3)д�����б����ṹ�����ܷ���������Ӧ���ܷ���ˮ�ⷴӦ��D��ͬ���칹��Ľṹ��ʽ______________��(�����������칹��ֻ��д��3��)
(4)�ڵķ�Ӧ������______________��
(5)д��F��G�ķ�Ӧ����ʽ______________��
(6)����ɱ��� �Ʊ�
�Ʊ� �ĺϳ�·��(���Լ���ѡ)��______________
�ĺϳ�·��(���Լ���ѡ)��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ײʱ����ȫ�����з�����Ӧ��10NaN3��2KNO3=K2O��5Na2O��16N2��������˵������ȷ����( )
A.��ԭ�ԣ�NaN3 > N2
B.����65 g NaN3�μӷ�Ӧ�������ɵ�N2�����ʵ���Ϊ1.6 mol
C.ÿת��1 mol���ӣ������ɱ�״����N2�����Ϊ35.84 L
D.����ԭ��N�뱻������NΪ15 ��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�ء���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�

(1)ָ���Ӻ�������ȡI2��ʵ��������ƣ�
��____________����__________�ڵ����ӷ���ʽ__________����
(2)��ȡ��Ĺ����У��ɹ�ѡ����л��ܼ�����____����
A �ױ����ƾ� B ���Ȼ�̼����
C ���͡����� D ���͡�����
(3)Ϊʹ������I��ת��Ϊ����л���Һ��ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������ȱ�ٵ�������________��
(4)�Ӻ�����л��ܼ�����ȡ�⣬��Ҫ��������ָ����������װ���еĴ���֮��__________��

(5)�����������ʱ��ʹ��ˮԡ���ȵ�ԭ����_________________�����̬����________�С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʾ��Һ��Ũ�ȵķ���ͨ�������֣���Һ�����ʵ���������(w)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������ա�
(1)��10%(�ܶ�Ϊ1.01 g��cm��3)������������Һ���Ƴ�27.5 g 2% ������������Һ��
�ټ��㣺��________g 10%(�ܶ�Ϊ1.01 g��cm3)������������Һ�������Ϊ________mL�����________mLˮ(��ˮ��1 g��cm��3)����ϡ�͡�
����ȡ����________mL��Ͳȡ10% �������ƣ���ȡʱ����Ҫ����Ͳ________����ˮƽ��Ȼ�����ձ����________mL��Ͳ��ȡ����ˮҲע���ձ��
���ܽ⣺��________��������Һ������ȣ�����27.5 g 2% ������������Һ��
(2)��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ����ϡ�ͳ�3 mol��L��1��ϡ����100 mL���ش��������⣺
����ҪȡŨ����________mL��
�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����__________________________(����ĸ����ͬ)��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�������
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
(3)ʵ����������1 mol��L��1������������Һ��1 mol��L��1��������Һ��100mL��
��Ҫ��������������Һ������������ƽ��ȡ�������ƹ���ʱ����ƽ����Ϊ________��
A��4.0 g����������B��4.00 g�������� C����4.0 g
������������������Һ��������Һ�ĸ��������У������Բ�ͬ����__________��
A����������ȡ������B���ܽ��ϡ�� ��C����Һ��ϴ�ӡ���D������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com