
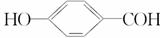
���ǵؿ��к�������Ԫ�ء�
(1)��Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ________����
(2)H2O�����ڵ�O��H�������Ӽ�ķ��»����������ǿ��������Ϊ
_________________________________________________________________��
 �ķе��
�ķе��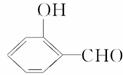 �ߣ�ԭ����_______________________________
�ߣ�ԭ����_______________________________
(3)H������H2O�γ�H3O����H3O����Oԭ�Ӳ���________�ӻ���H3O����
H��O��H���DZ�H2O��H��O��H���Ǵ�ԭ��Ϊ______________________
(4)CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊa g��cm��3��
NA��ʾ�����ӵ���������CaO�������Ϊ________cm3��
����
���������⿼�����ʽṹ�����ʣ����ڿ��鿼����ԭ�Ӻ�������Ų�����ѧ
���������֪ʶ�������Ӧ��������(1)��ԭ�ӻ�̬ԭ�ӵĺ�������Ų�ʽΪ
1s22s22p4��2p�������2������δ�ɶԡ�(2)������ڷ��Ӽ����������Ȼ�ѧ
���������ȷ��»���ǿ��(3)H3O����Oԭ��Ϊsp3�ӻ���(4)��1������Ϊ�о�
����1���������4��Ca2����4��O2��������m������V����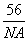 ��4��aV��
��4��aV��
V��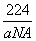 ��
��
�𰸡�(1)2��(2)O��H������������»�����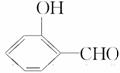 �γɷ�������
�γɷ�������
������ �γɷ��Ӽ���������Ӽ����ʹ����
�γɷ��Ӽ���������Ӽ����ʹ����
������������(3)sp3��H2O��Oԭ����2�Թµ��Ӷԣ�H3O����Oԭ��ֻ��
1�Թµ��Ӷԣ��ų�����С��(4)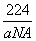
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
2CO(g)��O2(g)===2CO2(g)����H����566 kJ��mol��1
Na2O2(s)��CO2(g)===Na2CO3(s)�� O2(g)����H����226 kJ��mol��1
O2(g)����H����226 kJ��mol��1
���������Ȼ�ѧ����ʽ�жϣ�����˵����ȷ����(����)
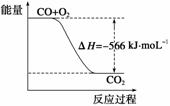
A��CO��ȼ����Ϊ283 kJ
B����ͼ�ɱ�ʾΪCO����CO2�ķ�Ӧ���̺�������ϵ
C��2Na2O2(s)��2CO2(s)===2Na2CO3(s)��O2(g) ��H>��452 kJ��mol��1
D��CO(g)��Na2O2(s)��Ӧ�ų�509 kJ����ʱ������ת����Ϊ6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����(C10H16)��һ����Ҫ��֬����������ṹ�߶ȶԳƣ�����ͼ��ʾ�����������±�ط���ȡ����Ӧ������һ��һ������(C10H14ClBr)��ͬ���칹����Ŀ��(����)
A��4�� B��6�� C��8�� D��10��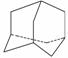
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��ͬ��Ԫ�ء�
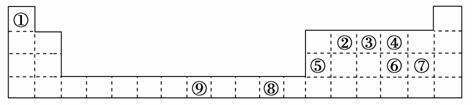
��ش��������⣺
(1)��������d����Ԫ����________(����)��Ԫ�آ��ԭ�Ӽ۵����Ų�ʽ��____________��
(2)�ۺ͢��γɵij���������ľ���������________���ڢۢܵĵ縺�ԣ�________��________��________(��Ԫ�ط��ű�ʾ)���ж�������_________________________________________________________________��
(3)ijԪ�ص����������Ų�ʽ(�۵����Ų�ʽ)Ϊnsnnpn��1����Ԫ��Ϊ���ڱ��е�________(����)����Ԫ����Ԫ�آ��γɵĻ�����X��������ˮ��ԭ����__________________________________________________________________
_________________________________________________________________��
(4)�ڢ��γ�һ�ֳ�Ӳ����ĥ�����µ��������ǽ������ϣ����仯ѧʽΪ________�����۵�Ƚ��ʯ��________(��ߡ��͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ͨ�����������Ծ������ʽ���ڣ���һ�־����ֿ�������������ͬ�Ļ����ṹ��Ԫ���ɣ���Щ�����ṹ��Ԫ�ڽṹ��ѧ�б�������������֪ij���������ɸơ��ѡ�������Ԫ����ɵľ��壬�侧���ṹ��ͼ��ʾ��������ʵĻ�ѧʽΪ (����)��
A��Ca4TiO3 B��Ca4TiO6
C��CaTiO3 D��Ca8TiO12
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֱ�ȡ1 mol�����ǽ����������飺
(1)������Ӧʱ������Ag�����ʵ���Ϊ________mol����Ӧ�������DZ�Ϊ__________________����ṹ��ʽ��_____________________________��
(2)�����ᷴӦ���������������Ͻ���ȫ������Ҫ________g���ᡣ
(3)��ʹ֮��ȫת��ΪCO2��H2O����������������ڱ�״����Ϊ________L����Ӧ�Ļ�ѧ����ʽ��____________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������г���ľ�Ǵ�����ζ����ľ�Ǵ����Է�ֹȣ�ݣ�ľ�Ǵ��Ľṹ��ʽΪ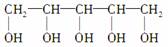 �������й�ľ�Ǵ���˵���У���ȷ����(����)
�������й�ľ�Ǵ���˵���У���ȷ����(����)
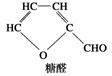
A��ľ�Ǵ���һ�ֵ��ǣ����ܷ���ˮ�ⷴӦ
B��ľ�Ǵ����ܽ���ˮ���ܷ���������Ӧ
C��ľ�Ǵ��ڿ�ǻ���ױ�����Ϊ��
D��ľ�Ǵ���ȥ������ˮ�ɵÿ�ȩ(�ṹ����ͼ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ������н������·�Ӧ��X2(g)+Y2(g) 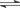 2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п�����
2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п�����
A�� ZΪ0.3mol/L B�� Y2Ϊ0.4mol/L
C�� X2Ϊ0.2mol/L D�� ZΪ0.4mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������£�һ���ܴ����������������(����)
A����ɫ����ˮ��Һ�У�K����Ba2����I����MnO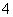
B�����д���NO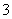 ��ˮ��Һ�У�NH
��ˮ��Һ�У�NH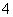 ��H2PO4����SO
��H2PO4����SO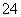 ��PO43��
��PO43��
C��c(HCO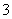 )��0.1 mol·L��1����Һ�У�Na����K����CO
)��0.1 mol·L��1����Һ�У�Na����K����CO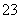 ��Br��
��Br��
D��������Һ�У�ClO����S2����SO32����Na��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com