

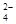 +2Br����2�֣�
+2Br����2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
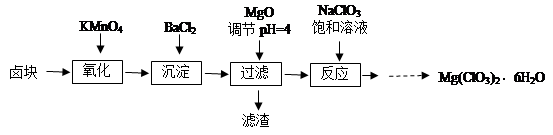
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
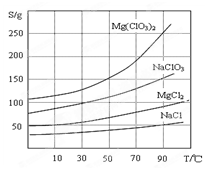
 Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�
 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________��
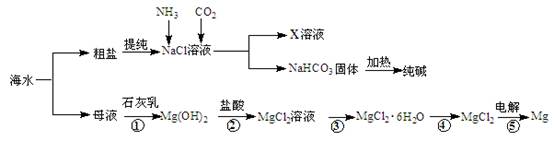
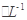 �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4�� ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
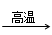 4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
 4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B���٢ڢ� | C���٢ڢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һ����ȡ������ | B����ȡ������Һ |
| C����Һ��������ȡ | D��������ȡ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
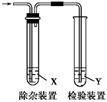
| | ��ϩ���Ʊ� | �Լ�X | �Լ�Y |
| A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
| B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
| C | C2H5OH��ŨH2SO4������170 �� | NaOH��Һ | KMnO4������Һ |
| D | C2H5OH��ŨH2SO4������170 �� | NaOH��Һ | Br2��CCl4��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com