
 ����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��
����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��| ʵ����� | 1 | 2 | 3 | 4 |
| ����������Һ�������mL�� | 20.05 | 20.00 | 22.10 | 19.95 |
���� ��1�����ݳ������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���ȷ��ʵ��������
��2����Ҫ�������ϴ�����ݣ�
���ȿ������ݵĺ����ԣ������к͵ζ�ԭ��������c��NaOH��=$\frac{c��HCl��V��HCl��}{V��NaOH��}$���㣻
��3������C�����⣩�T$\frac{C��������V������}{V�����⣩}$������
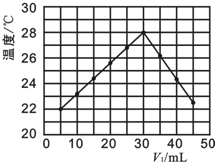
����4��A����ͼʾ�۲���ʼ�¶ȼ�Ϊʵ��ʱ�����¶ȣ�
B��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL���Դ˼���NaOHŨ�ȣ�Ȼ�������Һ�еijɷ��ԱȽ�����Ũ�ȣ�
C��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL���Դ˼���NaOHŨ�ȣ�
D������������ȣ�
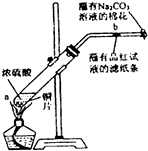
��� �⣺��1�����Ʋ����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ����Ũ���ᵹ���ձ������ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ���������в��������ձ�����ͷ�ιܡ�250mL����ƿ����Ͳ��
�ʴ�Ϊ����ͷ�ιܣ�250mL����ƿ��
��2���ٵ�3��ʵ���������ϴ�Ӧ��ȥ���ʴ�Ϊ��3��
������ʵ������ĵ��������Ƶ�������������ϴ�����Ҫ��ȥ��V��NaOH��=$\frac{20.05+20.00+19.95}{3}$mL=20.00mL����c��NaOH��=$\frac{c��HCl��V��HCl��}{V��NaOH��}$=$\frac{20mL��0.040mol•L-1}{10.00mL}$=0.080 mol•L-1���ʴ�Ϊ��0.080 mol•L-1��
��3��������������δ��������ϴ�ζ��ܣ����ᱻϡ�ͣ�Ũ�Ƚ��ͣ�����V������ƫ����C�����⣩=$\frac{C��������V������}{V�����⣩}$��������֪C�����⣩ƫ��
�ʴ�Ϊ��ƫ��
����4����ʵ�鿪ʼʱ�¶���21�棬��A����
B��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL��
ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ�����n��
HCl+NaOH=NaCl+H 2O
1 1
1.0mol•L-1��0.03L n
��n=1.0mol•L-1��0.03L=0.03mol������Ũ��Ϊ$\frac{0.03mol}{0.02L}$=1.5mol/L����V1=40ʱ����������ʵ���Ϊ0.04mol��NaOH�����ʵ���Ϊ0.015mol�������������Һ��c��Na+����c��Cl-����
��B��ȷ��
C��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL��
ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ�����n��
HCl+NaOH=NaCl+H2O
1 1
1.0mol•L-1��0.03L n
��n=1.0mol•L-1��0.03L=0.03mol������Ũ��Ϊ$\frac{0.03mol}{0.02L}$=1.5mol/L����C����
D������������ȣ��ų�������ƫС�����ܵõ���ͼ��ʵ��������D����
��ѡB��
���� ���⿼��������к͵ζ���������ѧ���㼰����������Ŀ�Ѷ��еȣ�ע�������к͵ζ��IJ����������ζ����ķ��������뼼�ɣ���������������ѧ�����Ӧ����ѧ֪ʶ���ʵ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| t/min | 2 | 4 | 7 | 9 |
| n��Y��mol | 0.12 | 0.11 | 0.10 | 0.10 |
| A�� | ��Ӧǰ2min��ƽ������x��Z��=2.0��10-3mol•L-1min-1 | |
| B�� | �����������䣬��ƽ����ϵ���ٳ���0.16mol����X����ԭƽ����ȣ��ﵽ��ƽ��ʱ������Y��ת��������X�������������Z������������� | |
| C�� | �����������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰv���棩��v������ | |
| D�� | ���������������䣬��ʼʱ����Һ�г���0.32mol����X��0.32mol����Y������ƽ��ʱ��n��Z����0.24mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ������Mg��OH��2��Һ�뱥��MgF2��Һ��ȣ�ǰ�ߵ�c��Mg2+���� | |
| B�� | 25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ | |
| C�� | 25��ʱ��Mg��OH��2������20mL 0.01mol/L�İ�ˮ�е�Ksp����20mL0.01mol/L NH4Cl��Һ�е�KspС | |
| D�� | 25��ʱ����Mg��OH��2������Һ�м���NaF��Һ��Mg��OH��2������ת��ΪMgF2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������μ��������ڣ�$\frac{c��N{H}_{4}^{+}��}{c��O{H}^{-}��}$������ | |
| B�� | �١��۵������Ϻ���Һ�д��ڣ�NH4++H2O?NH3•H2O+H+ | |
| C�� | �١�������Ȼ�ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��NH4+�� | |
| D�� | �١��۰������2��1��ϣ�c��NH4+����c��NH3•H2O����c��SO42-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ�������ϵ�����ı仯 | |||
| n��CO�� | n��H2O�� | n��CO2�� | n��H2�� | ||
| �� | 1 | 4 | 0 | 0 | �ų�������32.8 kJ |
| �� | 0 | 0 | 1 | 4 | �����仯��Q1 |
| �� | 1 | 1 | 2 | 1 | �����仯��Q2 |
| A�� | ���������з�Ӧ10min�ﵽƽ�⣬0��10minʱ���ڣ���CO��ʾ��ƽ����Ӧ���ʦԣ�CO��=4.0��10-2 mol/��L•min�� | |
| B�� | �������У���ʼʱ�ԣ�CO���������ԣ�CO������ | |
| C�� | ��ƽ�������ϵ�����ı仯��Q1=4Q2 | |
| D�� | ƽ��ʱ�������������CO������������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com