
| �� | A | B | C | D |
| ԭ�Ӻ��� | ˫�� | ��� | ��� | ��� |
| �������� | -1 | 0 | +1 | 0 |
���� A��˫��10���������Ҵ�һ����λ����ɣ�B�Ƕ��10���ӷ��ӣ����ɼ��Լ����ɵ�4ԭ�ӷ��ӣ���B��NH3��A��C��Ӧ������B��D����C��NH4+��D��H2O��
��1������һ����������1���Ҽ���һ��˫������1���Ҽ���1���м������㣻
��2�����������ᷴӦ�����Ȼ�泥��Ȼ�������ӻ�����������Ӽ������ۼ�����λ����
��3�����Ӽ����ʹ���ʵ��ܽ��Խϴ�
��� �⣺A��˫��10���������Ҵ�һ����λ����ɣ�B�Ƕ��10���ӷ��ӣ����ɼ��Լ����ɵ�4ԭ�ӷ��ӣ���B��NH3��A��C��Ӧ������B��D����C��NH4+��D��H2O��
��1��1��NH3�����к���3���������������3���Ҽ����ʴ�Ϊ��3��
��2�����������ᷴӦ�����Ȼ�泥�����ʽΪNH4Cl��NH4Cl�����ӻ�����������Ӽ���笠������д��ڵ��⼫�Թ��ۼ�����λ����
�ʴ�Ϊ��NH4Cl��ABD��
��3��������ˮ����֮����������ʹ�ð�����������ˮ��
�ʴ�Ϊ�����Ӽ������
���� ���⿼���˳���10���������漰��ѧ����������ѶȲ�������ȷ���ǽ���Ĺؼ�������ʱҪ��dz���10��������


 �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
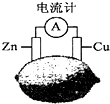
| A�� | ������ת��Ϊ��ѧ�� | B�� | ������ͭƬ���������ߵ�п | ||
| C�� | һ��ʱ���пƬ�������� | D�� | ͭƬһ�����ʱ���ɫ������Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
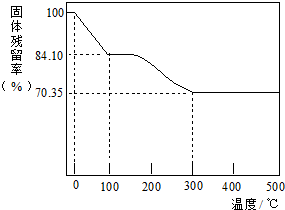
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����NMR�����������źŷ壬ǿ��֮��Ϊ3��1��
����NMR�����������źŷ壬ǿ��֮��Ϊ3��1��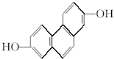 ���л����NMR���Ϲ۲�������ǿ��֮��Ϊ1��1��1��1��1��
���л����NMR���Ϲ۲�������ǿ��֮��Ϊ1��1��1��1��1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪V��W��X��Y��Z��ԭ������һ������Ķ���������Ԫ�أ�V��Y�����ڱ��е����λ�������ʾ��VԪ������Ԫ�����γ�������ɫ���壬X�ǵؿ��к��������Ľ���Ԫ�أ���ش��������⣺
��֪V��W��X��Y��Z��ԭ������һ������Ķ���������Ԫ�أ�V��Y�����ڱ��е����λ�������ʾ��VԪ������Ԫ�����γ�������ɫ���壬X�ǵؿ��к��������Ľ���Ԫ�أ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | �� | 23 | 24 |
| C2H6 | C2H6O | C2H4O2 | C3H8 | C3H8O | C3H6O2 | C4H10 | �� | M | N |
| A�� | MΪC9H20O | B�� | NΪC9H18O2 | ||
| C�� | M��N���ɷ����ӳɷ�Ӧ | D�� | M��N�����ܷ���ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
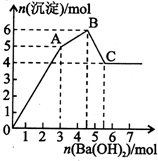 ��FeCl3��Al2��SO4��3�Ļ����Һ����μ���Ba��OH��2��aq�����γɳ������������ͼ��ʾ���������������ܽ��pH�����±�����֪��ƫ���ᱵ������ˮ���������ƶ���ȷ���ǣ�������
��FeCl3��Al2��SO4��3�Ļ����Һ����μ���Ba��OH��2��aq�����γɳ������������ͼ��ʾ���������������ܽ��pH�����±�����֪��ƫ���ᱵ������ˮ���������ƶ���ȷ���ǣ�������| �������� | ��ҺpH | |||
| ��ʼ���� | ������ȫ | ������ʼ�ܽ� | ������ȫ�ܽ� | |
| Fe��OH��3 | 2.3 | 3.4 | ||
| Al��OH��3 | 3.3 | 5.2 | 7.8 | 12.8 |
| A�� | ��ͼ����ԭ��Һ��c��Cl-��=c��SO42-�� | |
| B�� | OA�β����ij���ΪBaSO4��Fe��OH��3 | |
| C�� | AB�ο��ܷ����ķ�Ӧ�ǣ�2SO42-+2Ba2++Al3++30H-=2BaSO4k+Al��OH��3�� | |
| D�� | C����Һ�ʼ��Ե�ԭ����AlO2-ˮ�⣬�����ӷ���ʽΪ��AlO2-+2H2O=Al��OH��3+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
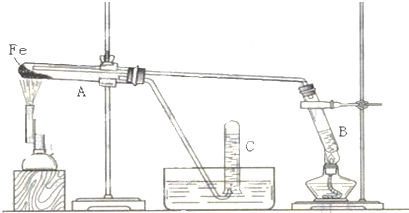
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��һ��������Mg-Al�Ͻ�Ͷ�뵽100mLһ�����ʵ���Ũ�ȵ�HCl�У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol/LNaOH��Һ�����������ɳ������������NaOH��Һ�������ϵ����ͼ��ʾ��
��һ��������Mg-Al�Ͻ�Ͷ�뵽100mLһ�����ʵ���Ũ�ȵ�HCl�У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol/LNaOH��Һ�����������ɳ������������NaOH��Һ�������ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com