
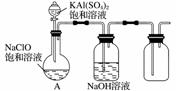
NaClO��KAl(SO4)2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
(1)NaClO��ҺpH��7�������ӷ���ʽ��ʾԭ��__________________________________
________________________________________________________________________��
(2)����NaClO�������Ʋ⣬��ֽ���м���NaClO��Һ��Ŀ����________________________________________________________________________��
(3)ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl(SO4)2��Һ��Ϸ�Ӧ��ʵ�顣��������ƿ�м��뱥��KAl(SO4)2��Һ�����������İ�ɫ��״��������Ӧ�����ӷ���ʽ��________________________________________________________________________
________________________________________________________________________��
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̬Siԭ���У�����ռ�ݵ�����ܲ����Ϊ________�����ܲ���е�ԭ�ӹ����Ϊ________��������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬ�¶ȡ���ͬŨ���µİ�����Һ����pH��С�����˳����ͼ��ʾ��ͼ�Т٢ڢۢܢݴ��������ʿ��ֱܷ�Ϊ(����)
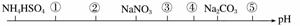
A��NH4Cl��(NH4)2SO4��CH3COONa��NaHCO3��NaOH
B��(NH4)2SO4��NH4Cl��CH3COONa��NaHCO3��NaOH
C��(NH4)2SO4��NH4Cl��NaOH��CH3COONa NaHCO3
D��CH3COOH��NH4Cl��(NH4)2SO4��NaHCO3��NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ��H2SO3??HSO ��H���ĵ��볣��Ka��1��10��2mol·L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kh��______mol·L��1������NaHSO3��Һ�м���������I2������Һ��
��H���ĵ��볣��Ka��1��10��2mol·L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kh��______mol·L��1������NaHSO3��Һ�м���������I2������Һ��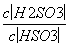 ��________(���������С�����䡱)��
��________(���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����й��ڵ������Һ���ж���ȷ����(����)
A����pH��12����Һ�У�K����Cl����HCO ��Na�����Դ�������
��Na�����Դ�������
B����pH��0����Һ�У�Na����NO ��SO
��SO ��K�����Դ�������
��K�����Դ�������
C����0.1 mol·L��1һԪ��BOH��Һ��pH��10������֪BOH��Һ�д���BOH===B����OH��
D����0.1 mol·L��1һԪ��HA��Һ��pH��3������֪NaA��Һ�д���A����H2O??HA��OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4_______________________________________________________________________��
(2)H2CO3_______________________________________________________________________��
(3)Ca(OH)2____________________________________________________________________��
(4)Fe(OH)3_____________________________________________________________________��
(5)NH3·H2O____________________________________________________________________��
(6)NaCl________________________________________________________________________��
(7)BaSO4______________________________________________________________________��
(8)NaHSO4_____________________________________________________________________��
(9)NaHCO3_____________________________________________________________________��
(10)NaHSO4(����)______________________________________________________________��
(11)Al2O3(����)_______________________________________________________________��
(12)CH3COOH_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������Ӧ�����ӷ���ʽ��ȷ����(����)
A����NaAlO2��Һ��ͨ������CO2��AlO ��CO2��2H2O===Al(OH)3����HCO
��CO2��2H2O===Al(OH)3����HCO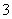
B�����ˮ�еμӱ��͵��Ȼ�����Һ��Fe3����3H2O===Fe(OH)3����3H��
C����̼���Ⱶ��Һ�м������������������Һ��Ba2����2HCO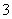 ��2OH��===BaCO3����CO
��2OH��===BaCO3����CO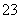 ��2H2O
��2H2O
D����FeCl2��Һ�м�������K3[Fe(CN)6]��Һ��4Fe2����2[Fe(CN)6]4��===Fe4[Fe(CN)6]2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��һ�־��й���ζ�ĺϳ����ϣ���ͼΪ�ϳ�X�����̡�E�������г�����һ���л��̼��������������ֱ�Ϊ52.17%��13.04%������Ϊ������������֪����Է�������Ϊ46���˴Ź���������ʾ����������ԭ�ӣ��Ҹ�����Ϊ1��2��3��
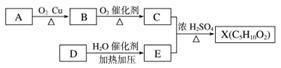
�����������Ϣ���ش��������⣺
(1)A�����й����ŵ������ǣ�____________��E�Ľṹ��ʽ�ǣ�____________��
(2)D��E�Ļ�ѧ��Ӧ����Ϊ____________��Ӧ��
(3)����A��B��C��D��E��X���������У���Ϊͬϵ����ǣ�____________��
(4)C��һ��ͬ���칹��F���Է���ˮ�ⷴӦ��������Ӧ����F�Ľṹ��ʽΪ________________________________________________________________________��
(5)��ӦC��E��X�Ļ�ѧ����ʽΪ__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com