
ij�л�������A�Ľṹ��ʽ���£�
HO��C������HCHNHCH2CH��O��C��CH��
CH2CHCH2������������C���������������������������������� H2OH
����
O
��1��A����ʽ�� ��
��2��A��NaOHˮ��Һ�м��ȷ�Ӧ�õ�B��C��C�Ƿ��㻯���B��C�Ľṹ��ʽ��B�� �� C�� ���÷�Ӧ���� ��Ӧ��
��3�������£�C��ϡ�����ữ�õ�E��E�Ľṹ��ʽ�� ��
��4�������������У�������E������ѧ��Ӧ����(��д���) ��
��ŨH2SO4��ŨHNO3�Ļ��Һ ��CH3CH2OH (���) ��CH3CH2 CH2CH3
��Na ��CH3COOH (���)
��5��д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ��
�ٻ�������1��3��5-��ȡ����
�ڱ����ϵ�����ȡ�����ֱ�Ϊ�����ǻ��ͺ��У�C��O���ṹ�Ļ���
��C16H21O4N
HO��C������HCHNHCH2CH��OH
CH2CHCH2������
CH��C��ONa
��C���������������������������������� H2OH
����
O
(B)(C)
������ˮ��(����)
CH��C��OH
��C���������������������������������� H2OH
����
O
�� �Ȣ�
��(��4������Ҫ���E��ͬ���칹��)
����
O
HOOCCH3H3C
����
O
HOCOCH3H3C
CH2OCH
����
O
HOH3CCH2COH
����
OHOH3C


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
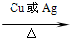 2CH3CHO+2H2O
2CH3CHO+2H2O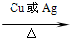 2CH3CHO+2H2O
2CH3CHO+2H2O| ˮԡ���� |
| ˮԡ���� |
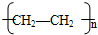 ��
��
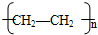 ��
�� CH3CH2Cl��
CH3CH2Cl�� CH3CH2Cl��
CH3CH2Cl�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
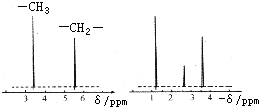 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |
| ���� |
| �� |
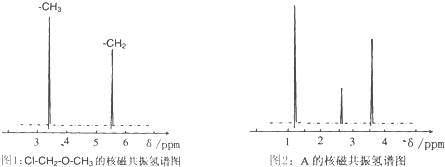
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
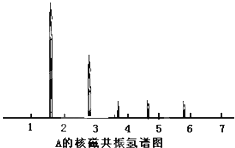 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮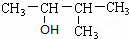
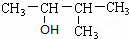




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com