
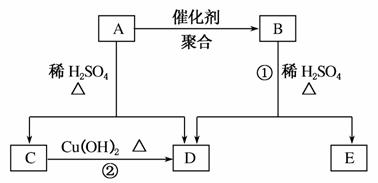
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ��
��֪R—CH===CHOH(ϩ��)���ȶ����ܿ�ת��ΪR—CH2CHO��
����������Ϣ�ش��������⣺
(1)A�ķ���ʽΪ________________��
(2)��Ӧ�ڵĻ�ѧ����ʽ��________________________________________________________________________��
(3)A�Ľṹ��ʽ��________________��
(4)��Ӧ�ٵĻ�ѧ����ʽ��________________________________________________________________________��
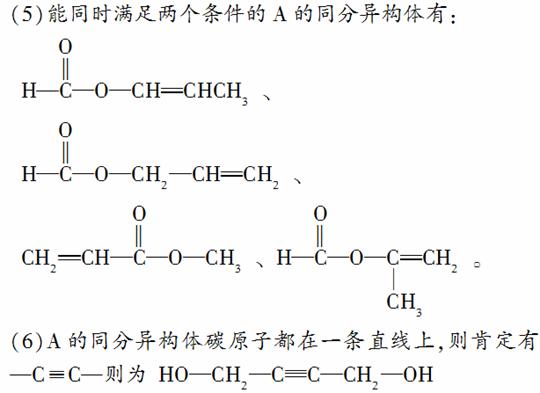
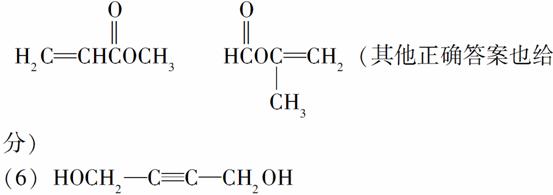
(5)A�ж���ͬ���칹�壬д���ĸ�ͬʱ����(ⅰ)�ܷ���ˮ�ⷴӦ(ⅱ)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��____________��____________��____________��____________��
(6)A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ________________��
������(1)A����Է�������Ϊ86��̼����������Ϊ55.8%����̼������Ϊ48����ԭ�Ӹ���Ϊ4�������������Ϊ7.0%�������ԭ�Ӹ���Ϊ6���Ӷ���֪����ԭ�Ӹ���Ϊ2������A�ķ���ʽΪC4H6O2��
(2)A����ϡH2SO4���������·�Ӧ��˵��A�к���������C����Cu(OH)2�ڼ��������·�Ӧ��D����C����ȩ����DΪ���ᣬ������֪�ɵ�CΪCH3CHO��DΪCH3COOH�����Է�Ӧ�ڵĻ�ѧ����ʽ�ǣ�CH3CHO��2Cu(OH)2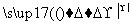 CH3COOH��Cu2O����2H2O��
CH3COOH��Cu2O����2H2O��

�𰸡�(1)C4H6O2



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵�����ʾ������ȷ����(����)
A����Ӧ������������������������ʱ���÷�Ӧ���ܷ���
B��ϡ��ǿ����ϡ��ǿ�Ӧ�ų������������к���
C����C(ʯī��s)===C(���ʯ��s)����H>0��֪ʯī�Ƚ��ʯ�ȶ�
D����101 kPa��25 ��ʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ������������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ2H2(g)��O2(g)===
2H2O(l)����H����285.8 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��Ӧ����101 kPaʱ��C(s)��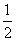 O2(g)===CO(g)����H1����110.5 kJ��mol��1
O2(g)===CO(g)����H1����110.5 kJ��mol��1
��ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l) ��H2����57.3 kJ��mol��1
���н�����ȷ����(����)
A����̼��ȼ�����æ�H3����ʾ����H3����H1
B����̼��ȼ�����æ�H3����ʾ����H3����H1
C��Ũ������ϡNaOH��Һ��Ӧ���к���Ϊ57.3 kJ��mol��1
D��ϡ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�������Ϊ57.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
3�������һ�ȴ�������(�����������칹)(����)
A��3�� B��4��
C��5�� D��6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������Ļ���ֻҪ������һ���������������ϣ���ȫȼ�պ����ɵ�CO2��H2O���Ǻ�������(����)
A��C2H2��C2H4 B��C2H4��C4H6
C��C2H6��C3H6 D��C6H6��C2H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E��������
��A��������14�����ӣ�����M���Ӳ�����2������
��B���õ�2�����Ӻ�����Ӳ�ṹ��Ne��ͬ
��C������һ����λ������ɣ��˵����Ϊ11
��D��������18�����ӣ���ʧȥ1������ʱ�ʵ�����
��E�������磬��������Ϊ1
����д��A��B��C��D��E�����ķ��ţ�________��
________��________��________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ����ȡ����Ӧ����(����)
A��C2H4��3O2 2CO2��2H2O
2CO2��2H2O
B��Zn��CuSO4===ZnSO4��Cu
C��NaCl��AgNO3===AgCl����NaNO3
D��CH2Cl2��Cl2 CHCl3��HCl
CHCl3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶������ܱ������з������·�Ӧ2M(g)��N(g)
 2E(g)������ʼʱ��ֻ����2 mol E���壬��ƽ��ʱ����������ѹǿ����ʼʱ������20%������ʼʱֻ����2 mol M��1 mol N�Ļ�����壬�ﵽƽ��ʱM��ת����Ϊ(����)
2E(g)������ʼʱ��ֻ����2 mol E���壬��ƽ��ʱ����������ѹǿ����ʼʱ������20%������ʼʱֻ����2 mol M��1 mol N�Ļ�����壬�ﵽƽ��ʱM��ת����Ϊ(����)
A��20% B��40% C��60% D��80%
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com