
����Ŀ������������ȹ��ɽ������ʼ���������ҽ�������й㷺��Ӧ�á�
��1����̬ԭ�����ļ۵����Ų�ʽΪ_____��
��2������ҩ��ɳ�����������Ҷ��Წ���Ľṹ��ʽ��ͼ��ʾ��
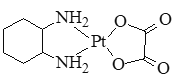
�ٷ����е�ԭ�ӹ�����ӻ�������_____��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_____��
��1 mol �Ҷ�������к��ЦҼ�����ĿΪ_____��
��3��̼����[La2(CO3)3]�������Ƹ���Ѫ֢��
��д����CO![]() ��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽ_____��
��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽ_____��
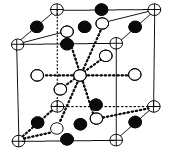
�������Ͻ�����ڴ��⣬�������Ļ�ѧʽΪLaNi5(H2)3������С�ظ��ṹ��Ԫ��ͼ��ʾ��![]() ��
��![]() ��
��![]() ���������е�������������ͼ��
���������е�������������ͼ��![]() ����������_____��
����������_____��
���𰸡� 3d84s2 sp3 N��O��C 7NA SO3 H2
����������1��Ni��ԭ������Ϊ28�������Ų�ʽΪ1s22s22p63s23p63d84s2����۵����Ų�ʽ��3d84s2����2���ٿ���ҩ��ɳ���������е�ԭ�Ӽ۵���������4��Ϊsp3�ӻ���C��N��O����ͬһ����Ԫ����ԭ���������μ�С��ͬһ����Ԫ�صĵ�һ����������ԭ������������������ڢ�A��Ĵ��ڵڢ�A��ģ��������һ�����ܴ�С˳����N��O��C���ڵ�������������˫����һ��������һ�������������1���Ҷ������һ��7��������2����������1 mol�Ҷ�������к�����������ĿΪ7NA����3���ȵ�������ָ������ͬ�۵�����Ŀ��ԭ����Ŀ�ķ��ӻ����ӣ���CO32-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ��SO3��(4)���ݾ�̯�����㣬![]() �ĸ���Ϊ8��1/8=1��
�ĸ���Ϊ8��1/8=1��![]() �ĸ���Ϊ8��1/2+1=5����ĸ���Ϊ8��1/4+2��1/2=3�����ݻ�ѧʽLaNi5(H2)3��֪��ͼ�С����������H2��
�ĸ���Ϊ8��1/2+1=5����ĸ���Ϊ8��1/4+2��1/2=3�����ݻ�ѧʽLaNi5(H2)3��֪��ͼ�С����������H2��


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�������ȷ����
��ˮ��Ħ��������18g
��0.5molH2�����Ϊ11.2L
��1 mol ˮ�к��� 2 mol ��� 1mol ��
��1mol �κ����ʶ�Լ����6.02��1023������
��0.5mol H2SO4���е�ԭ����ĿΪ3.5NA
��ֻ���ڱ�״���£������ͬ���κ����������ķ�������ͬ
����������Ϊ40%��������Һ��������ˮ��ϣ�������ҺŨ�ȴ���20%
�����ʵ���Ũ��Ϊ4mol/L ��������Һ���������ˮ��ϣ�������ҺŨ��С��2mol/L
A. �٢ۢܢݢߢ� B. �ڢݢߢ� C. �ݢߢ� D. �ݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������ȡ����Ӧ����(����)
A.��ϩ�ƾ���ϩB.����������ˮ��C.������������D.������Ũ��ˮ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������̵�����ã���ϳ�·������ͼ���£�
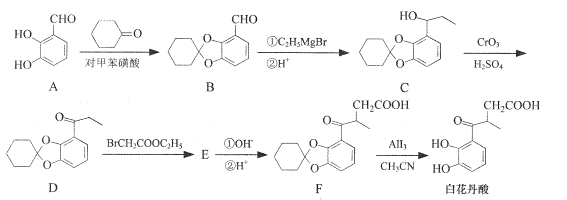
(1)A�еĺ�������������Ϊ__��_____��
(2)C-D�ķ�Ӧ����Ϊ ___��
(3)����������л���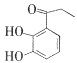 ��д��ͬʱ�������������ĸ��л����һ��ͬ���칹��Ľṹ��ʽ��____��
��д��ͬʱ�������������ĸ��л����һ��ͬ���칹��Ľṹ��ʽ��____��
�ٷ����������ֲ�ͬ��ѧ�������⣻����FeCl3��Һ�ܷ�����ɫ��Ӧ����1 mol�������������3 mol NaOH��Ӧ��
(4)E�Ľṹ��ʽΪ ___��
(5)��֪��![]() ��������֪ʶ����������Ϣд����
��������֪ʶ����������Ϣд����![]() ��CH3CH2OHΪԭ���Ʊ�
��CH3CH2OHΪԭ���Ʊ�![]() �ĺϳ�·������ͼ(�ϳ�·������ͼʾ�����������)____��
�ĺϳ�·������ͼ(�ϳ�·������ͼʾ�����������)____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¹���ѧ�ҹ�����1902 �꿪ʼ�о��ɵ���������ֱ�Ӻϳɰ�����Ӧԭ��Ϊ��N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJmol-1
2NH3(g) ��H=-92.4kJmol-1
��1���ں��º��������£���Ӧƽ����ϵ�г��뵪�����ﵽ��ƽ��ʱ��c(H2)��_________(����������������С�������������������ж�������ͬ)��c(N2)��c3(H2)��___________��
��2����ҵ�Ͽ���CH4��ˮ������������CH4(g)+H2O(g)![]() CO(g)+3H2(g)����200��ʱ2L���ܱ������У���1molCH4��1mol H2O(g)��ϣ���ƽ��ʱCH4 ��ת����Ϊ80%����200��ʱ�÷�Ӧ��ƽ�ⳣ��K=______________������һλС������
CO(g)+3H2(g)����200��ʱ2L���ܱ������У���1molCH4��1mol H2O(g)��ϣ���ƽ��ʱCH4 ��ת����Ϊ80%����200��ʱ�÷�Ӧ��ƽ�ⳣ��K=______________������һλС������
��3����ͼΪ�ϳɰ���Ӧ�ڲ�ͬ�¶Ⱥ�ѹǿ��ʹ����ͬ���������£���ʼʱ�����������������Ϊ1:3 ʱ��ƽ�������а���������������ֱ���vA(NH3)��vB(NH3)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��B ʱ�Ļ�ѧ��Ӧ���ʣ���vA(NH3)____ ( ����>����<������=��)vB(NH3)��

��4����ҵ�������ݳ��İ�����ϡ�������ա���ǡ������NH4HSO4������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����______________________��
��5��H2NCOONH4�ǹ�ҵ�ϳ����ص��м����÷�Ӧ�������仯��ͼ��ʾ����CO2�Ͱ��ϳ����ص��Ȼ�ѧ����ʽΪ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㡣
(1)0.7 mol H2O������Ϊ___________��
(2)___________mol H2O2����ԭ������0.2 mol H3PO4����ԭ������ȡ�
(3)ij��������Һ�к���3.01��1022��Na+������Һ��SO42�������ʵ�����___________��
(4)a mol ��������������������______________������NA��ʾ��
(5)��ij�Ȼ�����Һ�У���������ˮ���ӵ����ʵ���֮��Ϊa : b����Һ���ܶ�Ϊd g/mL,����Ȼ��Ƶ����ʵ���Ũ�ȿɱ�ʾΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ҫ��ҽҩ�м��壬��A��BΪԭ�Ϻϳɱ�����������F·�����£�
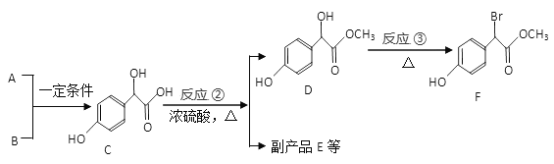
��1�� A �ķ���ʽΪC2H2O3��1molA �����ĺ���2.5 molCu(OH)2 ��������Һ��д����Ӧ���Ļ�ѧ����ʽ_______________________________��
��2��������C ��������������Ϊ______________����һ��������C ������NaOH ��Һ��Ӧ��1molC �������NaOH �����ʵ���Ϊ_________________mol��
��3����Ӧ���ķ�Ӧ����Ϊ___________�� ��д����Ӧ���Ļ�ѧ����ʽ_____________________________��
��4��E ����2 ������C���ɵĺ���3 ����Ԫ���Ļ����E �ķ���ʽΪ____________________��
��5������������F ������ͬ���칹��(�����������칹) ��__________�֣����к˴Ź��������������Ľṹ��ʽΪ____________________��
������һԪ��������� ��������ֻ��2 ��ȡ���� ����FeCl3 ��Һ����ɫ
��6�����������ϳ�·�ߡ��Ա�����Ϊԭ�������Լ���ѡ������Ʊ�A �ĺϳ�·�ߡ���֪RCH2COOH ![]() RCH(Cl)COOH______________________
RCH(Cl)COOH______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
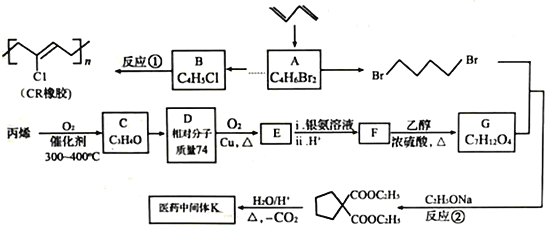
����Ŀ��ʯ���ѽ�����;�㷺����������ʯ���ѽ���Ϊԭ�Ϻϳ�CR��ҽҩ�м���K��·�ߣ�
��֪��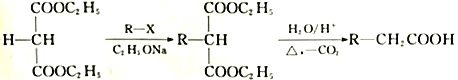
�ش��������⣺
��1��A��˳ʽ�칹��Ľṹ��ʽΪ________________��B�Ļ�ѧ������________________��C��������״�ṹ�������������Ϊ____________________________��
��2��K�Ľṹ��ʽΪ____________����Ӧ�١��ڵķ�Ӧ���ͷֱ�Ϊ_____________��_____________��
��3��D��E�Ľṹ��ʽ�ֱ�Ϊ_____________��_____________��
��4��F���Ҷ��������ۺϷ�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��5��д��ͬʱ��������������ҽҩ�м���K������ͬ���칹��Ľṹ��ʽ��_________________________________________________��
a.��E��Ϊͬϵ�� b.�˴Ź���������ʾΪ3���
��6����֪˫���ϵ���ԭ�Ӻ��ѷ���ȡ����Ӧ����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�B��д���ϳ�·�ߣ�_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������п�����ᷴӦ��ȡ�������������������䣬���д�ʩ��ʹ�÷�Ӧ����������ǣ� ��
A.�����¶�
B.��������п
C.��������Ũ��
D.��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com