
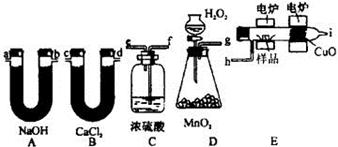
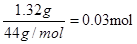 ��B����������0.54 g����ˮ��0.54g�����ʵ�����
��B����������0.54 g����ˮ��0.54g�����ʵ�����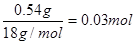 �������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��
�������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | �ڱ���������ص�������g�� | ����NaOH��Һ�������mL�� |
| 1 | 0.4080 | 18.20 |
| 2 | 17.10 | |
| 3 | 16.90 | |
| 4 | 17.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��Ũ���ḽ���������̣�NH3��HCl���巴Ӧ������NH4C1���� |
| B��Ũ���ḽ������������NH3��Ũ���������Ӧ |
| C���Ȼ�����Һ����ǣ�����Һһ����A1Cl3��Һ |
| D��ʪ��ĺ�ɫʯ����ֽ������NH3��ˮ��Һ�Լ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
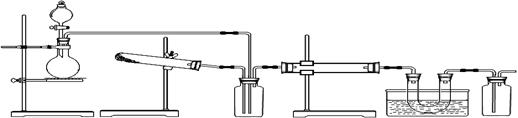
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����һ֧ʢ��2mL 2�� CuSO4��Һ���Թ��У����뼸��10����NaOH��Һ���ټ���1mL��ȩ��Һ�����Ⱥ���Կ�����ɫ������ͭ�������� |
| B��������������NaOH��Һ��ϲ���ˮԡ���ȣ����ɵõ�ˮ������Ҵ������� |
| C����������Ѿ�ˮ�⣬������������ϡ�������һ��ʱ�����NaOH��Һ�к����ᣬ�ټ���������Һ���� |
| D����һ֧�Թ��е���10�������飬�ټ���1mL 5����NaOH��Һ�����Ⱥ�μ���������Һ���ɹ۲쵽��dz��ɫ�廯���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com