
 ¹¤ŅµÉĻŅŌ»ĘĢśæóĪŖŌĮĻ£¬²ÉÓĆ½Ó“„·ØÉś²śĮņĖį£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¹¤ŅµÉĻŅŌ»ĘĢśæóĪŖŌĮĻ£¬²ÉÓĆ½Ó“„·ØÉś²śĮņĖį£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| ||
| ||


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ÉŁĮæµÄ¶žŃõ»ÆĮņĘųĢåĶØČėĄä°±Ė®ÖŠ£ŗSO2+NH3?H2OØTHSO3-+NH4+ |
| B”¢H218O ÖŠĶ¶ČėNa2O2¹ĢĢå£ŗ2H218O+2Na2O2ØT4Na++4OH-+18O2”ü |
| C”¢Ģ¼ĖįĒāøĘČÜŅŗÖŠ¼Ó¹żĮæ³ĪĒåŹÆ»ŅĖ®£ŗCa2++OH-+HCO3-ØTCaCO3”ż+H2O |
| D”¢Ģ¼ĖįÄʵÄĖ®½ā·“Ó¦£ŗCO32-+H3O+?HCO3-+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
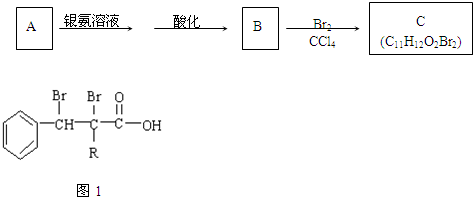
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £ØR”¢R”äæÉŅŌŹĒĢž»łŅ²æÉŅŌŹĒĒāŌ×Ó£©
£ØR”¢R”äæÉŅŌŹĒĢž»łŅ²æÉŅŌŹĒĒāŌ×Ó£©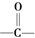 µÄŅģ¹¹Ģå¹²ÓŠøö
µÄŅģ¹¹Ģå¹²ÓŠøö²éæ““š°øŗĶ½āĪö>>
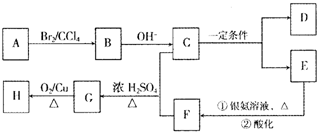
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó |
| “ņæŖµÆ»É¼Š£¬ĶØČėŅ»¶ĪŹ±¼äCO2£¬¹Ų±ÕµÆ»É¼Š£® | |
| “ņæŖ·ÖŅŗĀ©¶·»īČū£¬½«ÅØĻõĖį»ŗĀżµĪČėÉÕĘæÖŠ£¬¹Ų±Õ»īČū£® | ĪŽĆ÷ĻŌĻÖĻó£® |
| ¼ÓČČÉÕĘ棬·“Ó¦æŖŹ¼ŗóĶ£Ö¹¼ÓČČ£® | ¢ŁAÖŠÓŠŗģ×ŲÉ«ĘųĢå²śÉś£¬Ņ»¶ĪŹ±¼äŗó£¬ĘųĢåŃÕÉ«Öš½„±äĒ³£» BÖŠČÜŅŗ±ä×ŲÉ«£» CÖŠČÜŅŗ×ĻÉ«±äĒ³£®¢Ś·“Ó¦Ķ£Ö¹ŗó£¬AÖŠĪŽ¹ĢĢåŹ£Óą£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
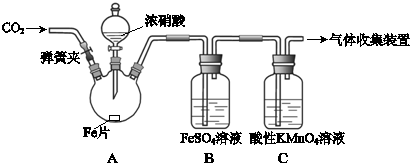
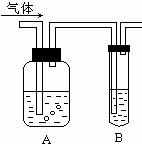 ČēĶ¼°ŃĘųĢå»ŗĀżĶعżŹ¢ÓŠ×ćĮæŹŌ¼ĮµÄŹŌ¼ĮĘæA£¬ŹŌ¹ÜB²»ÄܹŪ²ģµ½Ć÷ĻŌĻÖĻóµÄŹĒ£Ø””””£©
ČēĶ¼°ŃĘųĢå»ŗĀżĶعżŹ¢ÓŠ×ćĮæŹŌ¼ĮµÄŹŌ¼ĮĘæA£¬ŹŌ¹ÜB²»ÄܹŪ²ģµ½Ć÷ĻŌĻÖĻóµÄŹĒ£Ø””””£©| Ń”Ļī | ĘųĢå | AÖŠŹŌ¼Į | BÖŠŹŌ¼Į |
| A | SO2”¢CO2 | ĖįŠŌKMnO4ČÜŅŗ | ³ĪĒåŹÆ»ŅĖ® |
| B | Cl2”¢HCl | ±„ŗĶNaClČÜŅŗ | KIµķ·ŪČÜŅŗ |
| C | NH3”¢CO2 | ÅØĮņĖį | ·ÓĢŖŹŌŅŗ |
| D | CO2”¢HCl | ±„ŗĶNaHCO3ČÜŅŗ | NaAlO2ČÜŅŗ |
| AӢA | BӢB | CӢC | DӢD |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
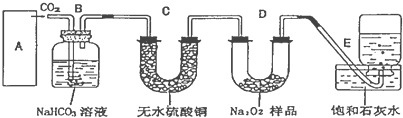
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 æÉĮ¬½ÓŌŚE“¦£¬ŌņCl2“Ó
æÉĮ¬½ÓŌŚE“¦£¬ŌņCl2“Ó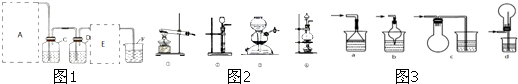
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com