
| A���ۢ� | B���ڢ� | C���ܢ� | D���٢� |
| n |
| V |


 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ��Һ���Ʋ��� | Ӱ�� | ԭ����� |
| A | ��2g I2���뵽98mL CCl4���ܽ���Ⱥ�����������Ϊ2%��I2��CCl4��Һ | ƫС | CCl4���ܶ� ����1g/mL |
| B | ����һ��Ũ�ȵ���Һʱ�����ձ��е���Һת�Ƶ�����ƿʱ������������ƿ�� | ƫС | ��������Һ�к������� |
| C | ����һ�����ʵ���Ũ�ȵ�������Һ������ʱ�����ˮ�����˿̶��ߣ������õι����������ˮ����ҡ�� | ��Ӱ�� | �����˶��� ��ˮ |
| D | ����һ�����ʵ���Ũ�ȵ�ʳ����Һ�����ݣ�ҡ�Ⱥ���Һ����ڿ̶��� | ƫ�� | Һ������ ƫС |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ӽ������� | B���Ǽ��Թ��ۼ� |
| C�����Ӽ� | D����λ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ڳ����¾����ױ����� |
| B�����ڿ�����ȼ������Na2O |
| C���ƿ��Ա�����ú���� |
| D��������Ȼ����ֻ���Ի��������ʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ϊ�˽�Na2CO3�����е�NaHCO3�����ȥ���Բ��ü��ȵķ��� |
| B��Na2CO3��Һ������Ca��OH��2��Һ��Ӧ����NaHCO3��Һ������ |
| C��Na2CO3��NaHCO3������CaCl2��Һ��Ӧ |
| D��������ͬ��Na2CO3 ��NaHCO3���������ᷴӦʱ��������CO2����Ҳ��ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
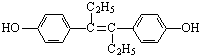
| A����ϩ�Ʒӵķ���ʽΪC18H20O2 |
| B����ϩ�Ʒӷ�����һ����16��̼ԭ�ӹ�ƽ�� |
| C����ϩ�Ʒ�Ϊ�����廯���� |
| D����ϩ�Ʒӿɷ����ӳɡ�ȡ�����������Ӿۡ�������������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ȳ�����д��ڵĦҼ������ڦм��� |
| B����Ϊ̼ԭ��֮���γɵĦҼ�Ҫ�Ȧм��ι̣�������ϩ������̼̼˫���ļ��ܲ��������������̼̼�������ܵ�2�� |
| C��������еĻ�ѧ���з����Ժͱ����� |
| D�����ɷ��Ӿ���������п���û�л�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������c��Na+������� |
| B���������ڶ���c��Na+��=2c��CO32-�� |
| C���������ڸ�������Ũ�Ⱦ���c��Na+����c��CO32-����c��OH-����c��HCO3-����c ��H+�� |
| D��������������Ũ�ȹ�ϵ��c ��H+��+c��Na+��=c��CO32-��+c��OH-��+c��HCO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH���ݣ��ۣ��ܣ��ڣ��٣��� |
| B��c��A2-�����ݣ��ڣ��ܣ��٣��ޣ��� |
| C����Һ��c��H+�����ޣ��٣��ܣ��ڣ��ۣ��� |
| D��c��H2A�����ޣ��ܣ��٣��ڣ��ݣ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com