
·ÖĪö Ō×Ó±ä³ÉŅõĄė×ÓŹ±µē×Ó²ćŹż²»±ä£¬Ō×Ó±ä³ÉŃōĄė×ÓŹ±µē×Ó²ćŹż¼õÉŁŅ»øö£¬Ķ¬Ņ»ÖÜĘŚµÄŅõŃōĄė×Óµē×Ó²ćŹż²»Ķ¬£¬Ķ¬Ņ»ÖÜĘŚµÄŅõĄė×ÓŗĶĻĀŅ»ÖÜĘŚµÄŃōĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬£»Ņ»°ć»īĘĆ½šŹōŌŖĖŲÓė»īĘĆ·Ē½šŹōŌŖĖŲŠĪ³ÉĄė×Ó¼ü£»Ķ¬ÖÖ·Ē½šŹōŌŖĖŲÖ®¼äŠĪ³É·Ē¼«ŠŌ¹²¼Ū¼ü£¬²»Ķ¬·Ē½šŹōŌŖĖŲÖ®¼äŠĪ³É¼«ŠŌ¹²¼Ū¼ü£»½į¹¹¶Ō³Ę£¬ÕżøŗµēŗɵÄÖŠŠÄÖŲŗĻ£¬ŌņĪŖ·Ē¼«ŠŌ·Ö×Ó£»½į¹¹²»¶Ō³Ę£¬ÕżøŗµēŗɵÄÖŠŠÄ²»ÖŲŗĻ£¬ŌņĪŖ¼«ŠŌ·Ö×Ó£¬¾Ż“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗA”¢Ć¾Ąė×Ó”¢ĀĮĄė×ÓŗĶÄŹµÄµē×Ó²ć½į¹¹ĻąĶ¬£¬±ČĀČĄė×ÓÉŁŅ»øöµē×Ó²ć£¬¹ŹA“ķĪó£»
B”¢ÄĘĄė×ÓŗĶ·śĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬£¬ĮņĄė×ÓŗĶė²Ō×Óµē×Ó²ć½į¹¹ĻąĶ¬£¬µ«ÄĘĄė×ÓŗĶ·śĄė×Ó±ČĮņĄė×ÓŗĶė²Ō×ÓÉŁŅ»øöµē×Ó²ć£¬¹ŹB“ķĪó£»
C”¢¼ŲĄė×Ó”¢øĘĄė×Ó”¢ĮņĄė×ÓŗĶė²ŗĖĶā¶¼ŹĒ18øöµē×Ó£¬ĖłŅŌµē×Ó²ć½į¹¹ĻąĶ¬£¬¹ŹCÕżČ·£»
D”¢ÄĘĄė×ÓŗĶĆ¾Ąė×Óµē×Ó²ć½į¹¹ĻąĶ¬£¬ĮņĄė×ÓŗĶĀČĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬£¬µ«ÄĘĄė×ÓŗĶĆ¾Ąė×Ó±ČĀČĄė×ÓŗĶĮņĄė×ÓÉŁŅ»øöµē×Ó²ć£¬¹ŹD“ķĪó£»
¹ŹŃ”C£»
K+”¢Ca2+”¢S2-ÖŠ£¬¾ßÓŠĻąĶ¬µē×Ó²ć½į¹¹£¬Ō×ÓŠņŹżŌ½Š”°ė¾¶Ō½“󣬹Ź°ė¾¶“óŠ”Ė³ŠņĪŖS2-£¾K+£¾Ca2+£¬¹Ź“š°øĪŖ£ŗS2-£¾K+£¾Ca2+£»
ŌŚO2”¢NH3”¢H2O2”¢£ØNH4£©2SO4”¢NaClÖŠ£¬O2 Ö»ŗ¬ÓŠ·Ē¼«ŠŌ¼ü£¬ŹōÓŚ·Ē¼«ŠŌ·Ö×Ó£»NH3Ö»ŗ¬¼«ŠŌ¼ü£¬ĪŖČż½Ē׶ŠĪ½į¹¹£¬½į¹¹²»¶Ō³Ę£¬ÕżøŗµēŗɵÄÖŠŠÄ²»ÖŲŗĻ£¬ĪŖ¼«ŠŌ·Ö×Ó£»H2O2 ŗ¬¼«ŠŌ¼üŗĶ·Ē¼«ŠŌ¼ü£¬½į¹¹²»¶Ō³Ę£¬ÕżøŗµēŗɵÄÖŠŠÄ²»ÖŲŗĻ£¬ĪŖ¼«ŠŌ·Ö×Ó£»£ØNH4£©2SO4 ŗ¬ÓŠ¹²¼Ū¼üŗĶĄė×Ó¼ü”¢ÅäĪ»¼ü£¬ŹōÓŚĄė×Ó»ÆŗĻĪļ£»NaClÖ»ŗ¬Ąė×Ó¼ü£¬ĪŖĄė×Ó»ÆŗĻĪļ£¬
£Ø1£©“ęŌŚ·Ē¼«ŠŌ¼üµÄ·Ö×ÓŹĒO2”¢H2O2£¬¹Ź“š°øĪŖ£ŗO2”¢H2O2£»
£Ø2£©“ęŌŚ¼«ŠŌ¼üµÄ·Ö×ÓŹĒNH3”¢H2O2£¬¹Ź“š°øĪŖ£ŗNH3”¢H2O2£»
£Ø3£©“ęŌŚ¼«ŠŌ¹²¼Ū¼üµÄĄė×Ó»ÆŗĻĪļŹĒ£ØNH4£©2SO4£¬¹Ź“š°øĪŖ£ŗ£ØNH4£©2SO4£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅõŃōĄė×Ó°ė¾¶“óŠ”±Č½Ļ”¢Ō×ӵĵē×Ó²ć½į¹¹”¢¼üµÄ¼«ŠŌŗĶ·Ö×ӵļ«ŠŌ£¬ÄŃ¶Č²»“ó£¬Ć÷Č·ŅõŃōĄė×ÓÖŠŗĖĶāµē×ÓµÄÅŲ»ŗĶ»ł±¾øÅÄīŹĒ½ā±¾ĢāµÄ¹Ų¼ü£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
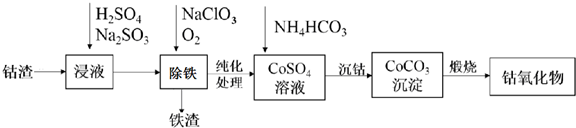
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł£¾¢Ś£¾¢Ū | B£® | ¢Ū£¾¢Ł£¾¢Ś | C£® | ¢Ū£¾¢Ś£¾¢Ł | D£® | ¢Ś£¾¢Ł£¾¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
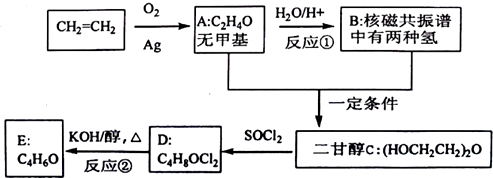
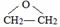 ”¢DClCH2CH2OCH2CH2Cl£®
”¢DClCH2CH2OCH2CH2Cl£®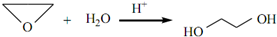 £»·“Ó¦¢ŚClCH2CH2OCH2CH2Cl+2KOH$”ś_{”÷}^{ŅŅ“¼}$CH2=CH-O-CH=CH2+2KCl+2H2O£®
£»·“Ó¦¢ŚClCH2CH2OCH2CH2Cl+2KOH$”ś_{”÷}^{ŅŅ“¼}$CH2=CH-O-CH=CH2+2KCl+2H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | »ģŗĻĘųĢåµÄŃ¹Ēæ | B£® | »ģŗĻĘųĢåµÄ×ÜĪļÖŹµÄĮæ | ||
| C£® | »ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ | D£® | v£ØB£©=3V£ØD£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ„Ī»Ź±¼äÄŚÉś³ÉnmolA2£¬Ķ¬Ź±ĻūŗÄ2n molAB | |
| B£® | ABµÄĻūŗÄĖŁĀŹµČÓŚA2µÄĻūŗÄĖŁĀŹ | |
| C£® | ČŻĘ÷ÄŚ£¬3ÖÖĘųĢåAB”¢A2”¢B2¹²“ę | |
| D£® | ČŻĘ÷ÖŠø÷×é·ÖµÄĢå»ż·ÖŹż²»Ėꏱ¼ä±ä»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÖŲ½šŹō”¢Å©Ņ©µČ»įŌģ³ÉĖ®ĢåĪŪČ¾ | |
| B£® | ×°ŹĪ²ÄĮĻÖŠµÄ¼×Č©”¢±½µČ»įŌģ³É¾ÓŹŅĪŪČ¾ | |
| C£® | CO»įµ¼ÖĀĖįÓźµÄŠĪ³É | |
| D£® | CO2µÄ“óĮæÅÅ·Å»į¼Ó¾ēĪĀŹŅŠ§Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | BeCl2 | B£® | H2O2 | C£® | COCl2 | D£® | SF6 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com