
(8·Ö)µ°æĒµÄÖ÷ŅŖ³É·ÖŹĒCaCO3£¬Ęä“ĪŹĒMgCO3”¢µ°°×ÖŹ”¢É«ĖŲµČ”£ĪŖ²ā¶ØĘäÖŠøʵÄŗ¬Į棬Ļ“¾»µ°æĒ£¬¼ÓĖ®Öó·ŠŌ¼5 min£¬ÖĆÓŚÕō·¢ĆóÖŠÓĆŠ”»šæ¾øÉ£¬ŃŠĻø”£
(1) ³ĘČ”0.3 g(ÉčĪŖ0.3000g)µ°æĒѳʷ£¬ÖĆӌ׶ŠĪĘæÖŠÖšµĪ¼ÓČėŅŃÖŖÅضČc(HCl)µÄŃĪĖį40.00 mL£¬¶ųŗóÓĆŠ”»š¼ÓČČŹ¹Ö®Čܽā£¬ĄäČ“ŗó¼Ó2µĪ¼×»ł³ČČÜŅŗ£¬ÓĆŅŃÖŖÅضČc(NaOH)»ŲµĪ£¬ĻūŗÄV(NaOH) L“ļÖÕµć”£
¢Ł (2·Ö)Š“³ö¼ĘĖćøĘŗ¬ĮæµÄĖćŹ½”£
¢Ś (1·Ö)¼ĘĖćµĆµ½µÄŹĒøʵÄŗ¬ĮæĀš£æ
¢Ū (1·Ö)ŹĒŌµ°æĒÖŠøʵÄŗ¬ĮæĀš£æ
(2) ³ĘČ”0.3 g(ÉčĪŖ0.3000 g)µ°æĒѳʷ£¬ÓĆŹŹĮæĒæĖįČܽā£¬Č»ŗó¼Ó(NH4)2C2O4µĆ³Įµķ£¬¾¹żĀĖ”¢Ļ“µÓ£¬³ĮµķČÜÓŚH2SO4ČÜŅŗ£¬ŌŁÓĆŅŃÖŖÅضČc(KMnO4)µĪ¶Ø(Éś³ÉMn2+ŗĶCO2)£¬ĻūŗÄV(KMnO4)L“ļµ½ÖÕµć”£
¢Ł (2·Ö)Š“³ö¼ĘĖćøĘŗ¬ĮæµÄĖćŹ½£»
¢Ś (2·Ö)“Ė·ØĒóµĆµÄøĘŗ¬ĮæĀŌµĶÓŚÉĻ·Ø”£ĪŖŹ²Ć“£æ
(1) ¢Ł
![]() (2·Ö)
(2·Ö)
¢ŚÓ¦ŹĒCa”¢MgµÄ×ÜĮæ(1·Ö)
¢Ū²»ŹĒ£¬ŅņĻ“µÓ”¢Öó·ŠŹ±³żČ„ĮĖÉŁĮæµ°°×ÖŹ£¬¶ųĖćŹ½ÖŠČ«°“Ca2+£¬¼“40.00 g/mol¼ĘĖć(1·Ö)
(2) ¢ŁCaCO3+2H+![]() Ca2++H2O+CO2”ü
Ca2++H2O+CO2ӟ
Ca2++C2O42-![]() CaC2O4”ż
CaC2O4”ż
CaC2O4+2H+![]() Ca2++H2C2O4
Ca2++H2C2O4
2MnO4+5H2C2O4+6H+![]() 2Mn2++10CO2ӟ+8H2O
2Mn2++10CO2ӟ+8H2O
¼“1 mol CaCO3”«1 mol Ca2+”«1 mol H2C2O4”«2/5 mol KMnO4
![]() (2·Ö)
(2·Ö)
¢Ś Ca2C2O4ĪŖÄŃČÜĪļ£¬ÄÜ“ÓČÜŅŗÖŠĶźČ«³Įµķ£¬ÓÖÄÜĶźČ«ČÜÓŚ¹żĮæĻ”ĮņĖį”£ŹµŃé½į¹ūøĘŗ¬ĮæµĶ£¬æÉÄÜŹĒMgC2O4Čܽā¶Č²»ŗÜŠ”Ö®¹Ź(ø½£ŗ18 ”ꏱMgC2O4?10H2OČܽā¶ČĪŖ0.03 g)£¬ÓĆĮņĖįČܽāŹ±£¬H2C2O4Įæ¼õÉŁÖ®¹Ź”£(2·Ö)


 ĢŲøß¼¶½ĢŹ¦µć²¦ĻµĮŠ“š°ø
ĢŲøß¼¶½ĢŹ¦µć²¦ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
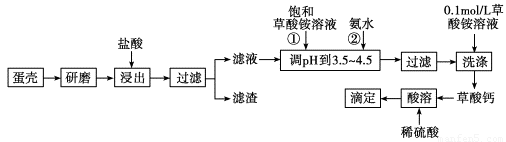
£Ø10·Ö£©µ°æĒµÄÖ÷ŅŖ³É·ÖŹĒCaCO3£¬Ęä“ĪŹĒSiO2”¢MgCO3¼°É«ĖŲµČŌÓÖŹ£¬²ā¶Øµ°æĒÖŠøʵÄŗ¬ĮæŹ±³£Éę¼°ČēĶ¼²Ł×÷²½Öč£¬ĘäÖŠ×īŗóµĪ¶Ø²Ł×÷ŹĒÓƱź×¼ĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶ØÉś³ÉµÄ²ŻĖį£¬ĶعżøĘÓė²ŻĖįµÄ¶ØĮæ¹ŲĻµ£¬æɼä½ÓĒó³öøʵÄŗ¬Į攣
׏ĮĻÖ§³Ö£ŗ¢Ł²ŻĖįµÄµēĄė·½³ĢŹ½ĪŖH2C2O4 H£«£«HC2O £»
¢ŚµĪ¶Ø¹ż³ĢµÄ·“Ó¦ĪŖH2C2O4£«MnO £«H£«ØD”śMn2£«£«CO2”ü£«H2O(Ī“ÅäĘ½)£»
¢ŪKsp(CaC2O4)£½2.510£9£¬Ksp(MgC2O4)£½8.610£5
øł¾ŻŅŌÉĻ²ÄĮĻ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ѳʷČÜÓŚŃĪĖįŗóµĆµ½µÄĀĖŌüÖ÷ŅŖŹĒ________”£
(2)¢Ł¢Ś²Ł×÷Ź±£¬¼ÓČė±„ŗĶ(NH4)2C2O4ČÜŅŗŗĶ°±Ė®µÄ×÷ÓĆŹĒ_______________£»
(3)ŹµŃéÖŠĶس£ÓĆ0.1 molL£1²ŻĖįļ§ČÜŅŗĻ“µÓ³Įµķ”£ČōŹµŃ鏱ÓĆĖ®Ļ“µÓ³Įµķ£¬ĒŅĆæ“ĪĻ“µÓŹ±ĻūŗIJŻĖįļ§ČÜŅŗŗĶĖ®µÄĢå»żĻąĶ¬£¬ŌņĆæ“ĪĻ“µÓŹ±ÓĆ²ŻĖįļ§ČÜŅŗĻ“µÓÓėÓĆĖ®Ļ“µÓĖšŹ§µÄÖŹĮæÖ®±ČĪŖ____________£»
(4)“ļµ½µĪ¶ØÖÕµćµÄĢŲÕ÷ĪŖ£»
(5)Čōæ¼ĀĒMgCO3µÄ“ęŌŚ£¬Ōņ“Ė·ØĒóµĆµÄøĘŗ¬ĮæĀŌ__________Źµ¼ŹÖµ£ØĢī>”¢=”¢<£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¼ŖĮÖŹ”¶«±±Ź¦“óø½ÖŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©µ°æĒµÄÖ÷ŅŖ³É·ÖŹĒCaCO3£¬Ęä“ĪŹĒSiO2”¢MgCO3¼°É«ĖŲµČŌÓÖŹ£¬²ā¶Øµ°æĒÖŠøʵÄŗ¬ĮæŹ±³£Éę¼°ČēĶ¼²Ł×÷²½Öč£¬ĘäÖŠ×īŗóµĪ¶Ø²Ł×÷ŹĒÓƱź×¼ĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶ØÉś³ÉµÄ²ŻĖį£¬ĶعżøĘÓė²ŻĖįµÄ¶ØĮæ¹ŲĻµ£¬æɼä½ÓĒó³öøʵÄŗ¬Į攣
׏ĮĻÖ§³Ö£ŗ¢Ł²ŻĖįµÄµēĄė·½³ĢŹ½ĪŖH2C2O4 H£«£«HC2O £»
¢ŚµĪ¶Ø¹ż³ĢµÄ·“Ó¦ĪŖH2C2O4£«MnO £«H£«ØD”śMn2£«£«CO2”ü£«H2O(Ī“ÅäĘ½)£»
¢ŪKsp(CaC2O4)£½2.510£9£¬Ksp(MgC2O4)£½8.610£5
øł¾ŻŅŌÉĻ²ÄĮĻ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ѳʷČÜÓŚŃĪĖįŗóµĆµ½µÄĀĖŌüÖ÷ŅŖŹĒ________”£
(2)¢Ł¢Ś²Ł×÷Ź±£¬¼ÓČė±„ŗĶ(NH4)2C2O4ČÜŅŗŗĶ°±Ė®µÄ×÷ÓĆŹĒ_______________£»
(3)ŹµŃéÖŠĶس£ÓĆ0.1 molL£1²ŻĖįļ§ČÜŅŗĻ“µÓ³Įµķ”£ČōŹµŃ鏱ÓĆĖ®Ļ“µÓ³Įµķ£¬ĒŅĆæ“ĪĻ“µÓŹ±ĻūŗIJŻĖįļ§ČÜŅŗŗĶĖ®µÄĢå»żĻąĶ¬£¬ŌņĆæ“ĪĻ“µÓŹ±ÓĆ²ŻĖįļ§ČÜŅŗĻ“µÓÓėÓĆĖ®Ļ“µÓĖšŹ§µÄÖŹĮæÖ®±ČĪŖ____________£»
(4)“ļµ½µĪ¶ØÖÕµćµÄĢŲÕ÷ĪŖ£»
(5)Čōæ¼ĀĒMgCO3µÄ“ęŌŚ£¬Ōņ“Ė·ØĒóµĆµÄøĘŗ¬ĮæĀŌ__________Źµ¼ŹÖµ£ØĢī>”¢=”¢<£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ¼ŖĮÖŹ”ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©µ°æĒµÄÖ÷ŅŖ³É·ÖŹĒCaCO3£¬Ęä“ĪŹĒSiO2”¢MgCO3¼°É«ĖŲµČŌÓÖŹ£¬²ā¶Øµ°æĒÖŠøʵÄŗ¬ĮæŹ±³£Éę¼°ČēĶ¼²Ł×÷²½Öč£¬ĘäÖŠ×īŗóµĪ¶Ø²Ł×÷ŹĒÓƱź×¼ĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶ØÉś³ÉµÄ²ŻĖį£¬ĶعżøĘÓė²ŻĖįµÄ¶ØĮæ¹ŲĻµ£¬æɼä½ÓĒó³öøʵÄŗ¬Į攣
׏ĮĻÖ§³Ö£ŗ¢Ł²ŻĖįµÄµēĄė·½³ĢŹ½ĪŖH2C2O4 H£«£«HC2O £»
¢ŚµĪ¶Ø¹ż³ĢµÄ·“Ó¦ĪŖH2C2O4£«MnO £«H£«ØD”śMn2£«£«CO2”ü£«H2O(Ī“ÅäĘ½)£»
¢ŪKsp(CaC2O4)£½2.510£9£¬Ksp(MgC2O4)£½8.610£5
øł¾ŻŅŌÉĻ²ÄĮĻ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ѳʷČÜÓŚŃĪĖįŗóµĆµ½µÄĀĖŌüÖ÷ŅŖŹĒ________”£
(2)¢Ł¢Ś²Ł×÷Ź±£¬¼ÓČė±„ŗĶ(NH4)2C2O4ČÜŅŗŗĶ°±Ė®µÄ×÷ÓĆŹĒ_______________£»
(3)ŹµŃéÖŠĶس£ÓĆ0.1 molL£1²ŻĖįļ§ČÜŅŗĻ“µÓ³Įµķ”£ČōŹµŃ鏱ÓĆĖ®Ļ“µÓ³Įµķ£¬ĒŅĆæ“ĪĻ“µÓŹ±ĻūŗIJŻĖįļ§ČÜŅŗŗĶĖ®µÄĢå»żĻąĶ¬£¬ŌņĆæ“ĪĻ“µÓŹ±ÓĆ²ŻĖįļ§ČÜŅŗĻ“µÓÓėÓĆĖ®Ļ“µÓĖšŹ§µÄÖŹĮæÖ®±ČĪŖ____________£»
(4)“ļµ½µĪ¶ØÖÕµćµÄĢŲÕ÷ĪŖ£»
(5)Čōæ¼ĀĒMgCO3µÄ“ęŌŚ£¬Ōņ“Ė·ØĒóµĆµÄøĘŗ¬ĮæĀŌ__________Źµ¼ŹÖµ£ØĢī>”¢=”¢<£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(±¾Ģā¼Ę8·Ö)»ĘĢśæóµÄÖ÷ŅŖ³É·ÖŹĒFeS2£¬Ä³ĮņĖį³§ŌŚ½ųŠŠ»ĘĢśæó³É·Ö²ā¶ØŹ±£¬Č”0.100 gѳʷŌŚæÕĘųÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉµÄSO2ĘųĢåÓė×ćĮæFe2(SO4)3ČÜŅŗĶźČ«·“Ó¦ŗó£¬ÓĆÅضČĪŖ0.0200 mol/LµÄK2Cr2O7±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄK2Cr2O7ČÜŅŗ25.00 mL”£ŅŃÖŖ£ŗSO2£«2Fe3£«£«2H2O£½SO42££«2Fe2£«£«4H£«£»Cr2O72££«6Fe2£«£«14H£«£½2Cr3£«£«6Fe3£«£«7H2O”£ (1)ѳʷ֊FeS2µÄÖŹĮæ·ÖŹżŹĒ(¼ŁÉčŌÓÖŹ²»²Ī¼Ó·“Ó¦)_____________________”£
(2)ČōČ¼ÉÕ6 g “æ¾»µÄFeS2²śÉśµÄSO2Č«²æ×Ŗ»ÆĪŖSO3ĘųĢåŹ±·Å³ö9.83 kJµÄČČĮ棬²śÉśµÄSO3ÓėĖ®Č«²æ»ÆŗĻÉś³ÉĮņĖįŹ±·Å³ö13.03 kJµÄČČĮ攣Š“³ö½Ó“„ŹŅÖŠ·¢Éś·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________”£
(3)ģŃÉÕ10 tÉĻŹö»ĘĢśæó£¬ĄķĀŪÉĻæÉÖʵĆ98%ÅØĮņĖį____________________t£¬SO2Č«²æ×Ŗ»ÆĪŖĮņĖįŹ±·Å³öµÄČČĮæŹĒ____________________kJ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com