
����Ŀ����A�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
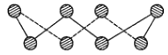
(1)S���ʵij�����ʽΪS8���价״�ṹ����ͼ��ʾ��Sԭ�Ӳ��õĹ���ӻ���ʽ��_____��
(2)ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����������Ҫ�����������O��S��Seԭ�ӵĵ縺���ɴ�С��˳��Ϊ___;
(3)Seԭ������Ϊ____�������M����ӵ��Ų�ʽΪ____��
(4)H2Se�����Ա�H2S___(����ǿ����������)����̬SeO3���ӵ����幹��Ϊ____��SO32-���ӵ����幹��Ϊ______��
(5)ZnS��ӫ���塢�����ϡ�Ϳ�ϡ����ϵ���ҵ��Ӧ�ù㷺������ZnS����ṹ����ͼ��ʾ���侧���߳�Ϊ540.0 pm���ܶ�Ϊ_________g��cm-3(��ʽ������)��aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ_____pm(��ʽ��ʾ)��

���𰸡�sp3 O>S>Se 34 3s23p63d10 ǿ ƽ�������� ������ 4.1 135![]() ��
��![]()
��������
(1)����S8�Ļ�״�ṹ֪��ÿ��Sԭ���γ�2��![]() ��������2�Թµ��Ӷԣ����ݼ۲���ӶԻ�������ȷ��Sԭ���ӻ���ʽ��
��������2�Թµ��Ӷԣ����ݼ۲���ӶԻ�������ȷ��Sԭ���ӻ���ʽ��
(2)ͬһ����Ԫ�أ����ϵ���Ԫ�صĵ縺�Լ�С��
(3)SeΪ34��Ԫ�أ�M���Ӳ�����18�����ӣ��ֱ�λ��3s��3p��3d�ܼ��ϣ�
(4)ͬ����Ԫ��ԭ�Ӱ뾶ԽС���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ��룬���Ծ�Խ����
���ݼ۲���ӶԻ�������ȷ����̬SeO3���ӵ����幹�͡�SO32-���ӵ����幹�ͣ�
(5)���þ�̯�����㾧���к��е�S2-��Zn2+��������=![]() �����ܶȣ����ݾ���aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ��Խ��ߵ�
�����ܶȣ����ݾ���aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ��Խ��ߵ�![]() ��
��
(1)����S8�Ļ�״�ṹ֪��ÿ��Sԭ���γ�2��![]() ��������2�Թµ��Ӷԣ�����ÿ��Sԭ�ӵļ۲���Ӷ�����4����Sԭ��Ϊsp3�ӻ���
��������2�Թµ��Ӷԣ�����ÿ��Sԭ�ӵļ۲���Ӷ�����4����Sԭ��Ϊsp3�ӻ���
��ˣ�������ȷ���ǣ�sp3��
(2)ͬһ����Ԫ�أ����ϵ���Ԫ�صĵ縺�Լ�С�����Ե縺���ɴ�С��˳����O>S>Se��
��ˣ�������ȷ���ǣ�O>S>Se��
(3)SeΪ34��Ԫ�أ�M���Ӳ�����18�����ӣ��ֱ�λ��3s��3p��3d�ܼ��ϣ����������M����ӵ��Ų�ʽΪ3s23p63d10��
��ˣ�������ȷ���ǣ�34��3s23p63d10��
(4)ͬ����Ԫ��ԭ�Ӱ뾶ԽС���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ��룬���Ծ�Խ�����ǽ�����S>Se������H2Se�����Ա�H2Sǿ����̬SeO3������Seԭ�Ӽ۲���Ӷ�����3+![]() ��(6-3��2)=3�Ҳ����µ��Ӷԣ����������幹��Ϊƽ�������Σ�SO32-������Sԭ�Ӽ۲���Ӷ���=3+
��(6-3��2)=3�Ҳ����µ��Ӷԣ����������幹��Ϊƽ�������Σ�SO32-������Sԭ�Ӽ۲���Ӷ���=3+![]() ��6+2-3
��6+2-3![]() 2��=4�Һ���һ�Թµ��Ӷԣ����������幹��Ϊ�����Σ�
2��=4�Һ���һ�Թµ��Ӷԣ����������幹��Ϊ�����Σ�
��ˣ�������ȷ���ǣ�ǿ��ƽ�������Σ������Σ�
(5)����ȫ���ھ����ڲ����þ����к��к��������4���������=![]() ��8+
��8+![]() ��6=4����=
��6=4����=![]() =
=![]() ��(540��10-10cm)3=4.1g/cm3�����ݾ���aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ��Խ��ߵ�
��(540��10-10cm)3=4.1g/cm3�����ݾ���aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ��Խ��ߵ�![]() ����aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ
����aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ![]() ��
��![]() ��540pm=135
��540pm=135![]() pm��
pm��
��ˣ�������ȷ���ǣ�4.1��135![]() ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CuCl�㷺Ӧ���ڻ�����ӡȾ����ҵ��ij�о�С���ô�ͭ(������Fe) Ϊԭ���Ʊ�CuCl2��2H2O���壬����CuCl2��2H2O�����Ʊ�CuCl��
(1)�Ʊ�CuCl2��2H2O���壺
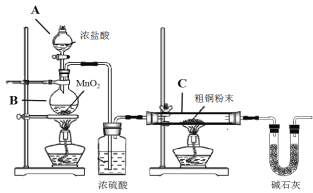
�� Bװ���з�����Ӧ�Ļ�ѧ����ʽ��_________________
�� װ��C�д�ͭ��ĩ(������Fe)�������ַ�Ӧʱ����������______________
�� ��Ӧ��ɺ�C�еĹ�����ϡ������ȫ�ܽ⣬�ٵ���pHֵ���ӣ���һϵ�в�����ɻ��CuCl2��2H2O���塣�ܽ�C�����������ʱ����ʱ���������˫��ˮ��Ŀ����__________
(2)��ȡCuCl��Ϊ����ȡCuCl������ͼ��ʾװ�ý���ʵ��(�г�������)��
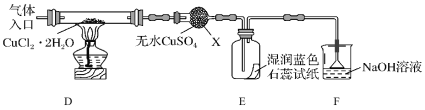
��֪��![]()
������X��������__________
��ʵ��������Ⱥ�˳����a��__________________________��e(������ı��)��
a�����װ�õ������Ժ����ҩƷ b��Ϩ��ƾ��ƣ���ȴ
c�����������������ͨ�����HCl d����ȼ�ƾ��ƣ�����
e��ֹͣͨ��HCl��Ȼ��ͨ��N2
��װ��F��NaOH��Һ��������___________________��
�� ��Ӧ������ȡ��CuCl��Ʒ����ʵ�飬�������к���������CuCl2��CuO���ʡ����������CuCl2���ʵ�ԭ����__________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý�������ʵ�飬ʵ������Ԥ�������һ�µ���

���е����� | ���е����� | Ԥ���������� | |
A | ���۵⻯����Һ | Ũ���� | ���������� |
B | ��̪��Һ | Ũ���� | ���������� |
C | �Ȼ�����Һ | Ũ��ˮ | �а�ɫ���� |
D | ʪ���ֽ�� | ������ˮ | ��ֽ����ɫ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ��������ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵ���Χ�����Ų�ʽ_____����λ�����ڱ�____����
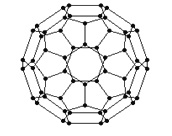
��2������ϩ���������ھ������õĹ�����ܣ���̫���ܵ�ص�Ӧ���Ͼ��зdz�������ǰ;������ϩ��C60���Ľṹ��ͼ��������̼ԭ�ӹ�����ӻ�����Ϊ________________��1mol C60��������������ĿΪ____________����
��3����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�أ�GaAs�������ӣ�CdS����Ĥ��صȣ�
�ٵ�һ�����ܣ�As____Ga����������������������=������
��SeO2���ӵĿռ乹��Ϊ_____��
��4������������NF3����һ����ɫ����ζ�����Ҳ���ȼ�����壬��̫���ܵ�������еõ��㷺Ӧ�ã�������ͭ�Ĵ���������F2������NH3��Ӧ�õ����÷�Ӧ��NH3�ķе�____����������������������=����HF�ķе㣬NH4F��������____���壮������ͭ��Һ�м��������ˮ�������������ӣ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о����֣�NOx��SO2����������Ҫ�ɷ֡�
��. NOx��Ҫ��Դ������β�����������û�ѧ����������ת��Ϊ���������ʡ�
��֪��N2(g)��O2(g) ![]() 2NO(g)����H����180 kJ��mol��1
2NO(g)����H����180 kJ��mol��1
2CO(g)��O2(g) ![]() 2CO2(g)����H����564 kJ��mol��1
2CO2(g)����H����564 kJ��mol��1
��1��2NO(g)��2CO(g)![]() 2CO2(g)��N2(g)����H��________.
2CO2(g)��N2(g)����H��________.
��2��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2 L���ܱ������У������¶Ⱥ�������䣬��Ӧ����(0��15 min)��NO�����ʵ�����ʱ��仯��ͼ��ʾ��
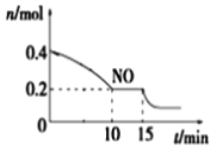
����֪��ƽ��ʱ����ķ�ѹ������������������ϵ����ѹǿ��T��ʱ�ﵽƽ�⣬��ʱ��ϵ����ѹǿΪp=20MPa����T��ʱ�÷�Ӧ��ѹ��ƽ�ⳣ��Kp ��_______��ƽ����������¶Ȳ��䣬���������г���NO��CO2��0.3mol��ƽ�⽫_____ (����������ҡ�����)�ƶ���
��15 minʱ�����ı���練Ӧ����������n(NO)��������ͼ��ʾ�ı仯����ı������������_____(�����)
A.����COŨ�� B.���� C.��С������� D.�������
��. SO2��Ҫ��Դ��ú��ȼ�ա�ȼ����������������Ǽ��ٴ����к�������Ⱦ�Ĺؼ���
��֪�������Ka1=2.0��10-2 Ka2=6.0��10-7
��3����ͨ������֤����NaHSO3��Һ�����Ե�ԭ��_________________________
��4����ͼʾ�ĵ��װ�ã��ɽ������е�NO��SO2ת��Ϊ����泥��Ӷ�ʵ�ַ����Ļ��������á�ͨ��NO�ĵ缫��ӦʽΪ____________________����ͨ���NO���Ϊ4.48L(�����)��������һ���缫ͨ���SO2��������Ϊ________g��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ���Ȼ�����SnCl4����һ����;�㷺���������м��壬ʵ���ҿ������ڵ������۵�231�棩��Cl2��Ӧ�Ʊ�SnCl4��װ����ͼ��ʾ����ش��������⣺

��֪��SnCl4�ڳ�ʪ�Ŀ����м���ˮ������SnO2xH2O��
���� | ��ɫ��״̬ | �۵�/�� | �е�/�� |
SnCl2 | ��ɫ���� | 246 | 652 |
SnCl4 | ��ɫҺ�� | -33 | 114 |
��1������a��������______��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2��װ��B��C��F��G��ʢ�ŵ�����Լ�����Ϊ______������ţ��Լ����ظ�ʹ�ã���
�ٱ���Na2CO3��Һ��NaOH��Һ��ŨH2SO4�ܱ���NaCl��Һ��H2O
��3����ȼ�ƾ���ǰ��Ҫ���е�һ��������______��
��4������ȥװ��C����D�з�������Ҫ����Ӧ��ѧ����ʽΪ______��
��5���õ��IJ����г�����SnCl2��ijʵ��С���õ��������ζ�������Ʒ��SnCl4�ĺ�����Sn2++I2=Sn4++2I-����ȷ��ȡ����Ʒmg������ƿ�У�������Ũ�����ܽ⣬�ټ�ˮϡ�ͣ�������Һ��ָʾ������0.1molL-1�����Һ�ζ����յ�ʱ��ƿ����Һ��ɫ�仯��______�������ı�Һ20.00mL�����Ʒ��SnCl4����������Ϊ______���ú�m�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ȼ�ϵ�أ���ͼ��ʾ������˵������ȷ����

A.�����ĵ缫��ӦʽΪO2+4e��+4H+=2H2O
B.�������缫1���������缫2
C.Na+���������ƶ�
D.NH3�ڵ缫1�Ϸ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ�ڹ�ҵ����Ҫ�������쵼�ߡ�����Ԫ���ȣ�ͭ���γɣ�1�ۺͣ�2�۵Ļ����
(1)д����̬Cu���ĺ�������Ų�ʽ___________________________________��
(2)��ͼ��ͭ��ij��������ľ���ʾ��ͼ����������Ļ�ѧʽΪ________��
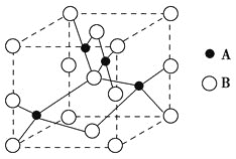
(3)������ͭ��Һ�еμӰ�ˮ��������ɫ�������ٵμӰ�ˮ�������պ�ȫ���ܽ�ɵõ�����ɫ��Һ�����������м��뼫�Խ�С���Ҵ�������������ɫ��[Cu(NH3)4]SO4��H2O�������������е�NH3ͨ��________������������Cu2����ϣ�NH3������Nԭ�ӵ��ӻ���ʽ��____����NH3���ӻ�Ϊ�ȵ������һ������________��
(4)CuO���۵��CuCl���۵�____(��ߡ��͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A~E���������ӵ�ʾ��ͼ��ṹ��ա�

(1)E�ķ���ʽ��_____________________��
(2)����ͬһ���ʵ���___________(�����)��
(3)��C��Ϊͬϵ�����___________(�����)��
(4)�����ʵ�������������ȫȼ��ʱ����![]() ������___________(�����,��ͬ);����������������ȫȼ��ʱ����
������___________(�����,��ͬ);����������������ȫȼ��ʱ����![]() ������_____________��
������_____________��
(5)��120��,![]() ��,A��C�ֱ�������
��,A��C�ֱ�������![]() ��ϵ�ȼ,��ȫȼ�պ�ָ�����ʼ״̬,�������û�б仯����______________(�����)��
��ϵ�ȼ,��ȫȼ�պ�ָ�����ʼ״̬,�������û�б仯����______________(�����)��
(6)C��ij��ͬϵ��ķ���ʽΪ![]() ,��һ�ȴ���ֻ��һ��,�����Ľṹ��ʽΪ______________________��
,��һ�ȴ���ֻ��һ��,�����Ľṹ��ʽΪ______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com