



 2H2O+O2����2�֣���������ȷ�𰸡�
2H2O+O2����2�֣���������ȷ�𰸡� 2H2O+O2��
2H2O+O2��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���ᴿ������ | ѡ�õ��Լ� | ��Ӧ���ӷ���ʽ |
| ��1�� Mg (Al) | | |
| ��2�� FeCl3(FeCl2) | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���� | ���� | ���� |
| A | �μ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I�� |
| B | ����ҺX ���ȵμ�ϡ���ᣬ�ٵμ�Ba(NO3)2��Һ | ���ְ�ɫ���� | ��ҺX ��һ������SO42- |
| C | �ýྻ��˿պȡ��Һ������ɫ��Ӧ | ����ʻ�ɫ | ԭ��Һ����Na������K+ |
| D | �μ�ϡNaOH��Һ����ʪ���ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽��(��Ҫ��д�������������) | Ԥ��ʵ������ͽ��� |
| ȡ������ɫ���壬 | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeCl3��Һ | B��AgNO3��Һ | C����ˮ | D��KMnO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������X�ijɷ���SiO2 |
| B��������ʯ����ֱ�����ڹ�����NaOH��Һ����ˣ��ɵõ���ɫ����Fe2O3 |
| C������ҺY�м��������NaOH��Һ�����˵õ��ij�������Ҫ�ɷ���Fe(OH)3��Mg(OH)2 |
| D����ҺY�е���������Ҫ��Mg2+��Al3+��Fe3+��H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� | �ж� |
| �� | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü�����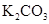 | |
| �� | ��ij��Һ�м������ᣬ�����������������м���BaCl2��Һ�а�ɫ���������� | ֤������Һ���� SO42�� | |
| �� | �������Һ�м���һ������ϡ������ȣ��ټ���һ����������������ͭ���ȡ� | ֤������ˮ����������� | |
| �� | 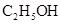 ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������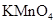 ��Һ ��Һ | �����Ƶ������Ƿ�Ϊ��ϩ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
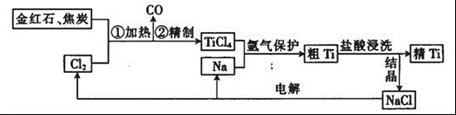


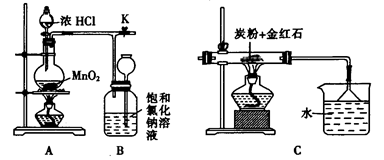
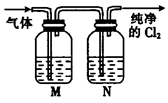
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| A��Ʒ����Һ | B����ɫʯ����Һ |
| C������KMnO4��Һ | D����ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com