


 ĘŚÄ©100·Ö“³¹Ųŗ£µķæ¼ĶõĻµĮŠ“š°ø
ĘŚÄ©100·Ö“³¹Ųŗ£µķæ¼ĶõĻµĮŠ“š°ø Š”ѧÄÜĮ¦²āŹŌ¾ķĻµĮŠ“š°ø
Š”ѧÄÜĮ¦²āŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŗĖÄÜ | B£®ÉśĪļÖŹÄÜ | C£®µēÄÜ | D£®»ÆѧÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®-69£®4 kJ/mol | B£®-45£®2kJ/mol |
| C£®+69£®4 kJ/mol | D£®+45£®2 kJ/mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 H2(g) £«
H2(g) £«  F2 (g) £½ HF (g) ”÷H = £269 kJ”¤mol£1
F2 (g) £½ HF (g) ”÷H = £269 kJ”¤mol£1 O2(g) £½ H2O (g) ”÷H = £242 kJ”¤mol£1
O2(g) £½ H2O (g) ”÷H = £242 kJ”¤mol£1²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
|
|
|
|
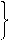 2CO(g)+O2(g) ="==" 2CO2(g)2H2(g)+O2(g) =2H2O(g)
2CO(g)+O2(g) ="==" 2CO2(g)2H2(g)+O2(g) =2H2O(g)²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®»Æѧ·“Ó¦±ŲČ»°éĖęÄÜĮæ±ä»Æ |
| B£®25”ę£¬101kPaĻĀ£¬l mol C8H18£ØŠĮĶé£©Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·Å³ö5518 kJČČĮæ,ĘäČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC8H18 (l)£«12.5O2 (g)£½8CO2 (g)£«9H2O (l)”÷H£½+5518kJ?mol£1 |
| C£®ĖįŗĶ¼īµÄÖŠŗĶ·“Ó¦¶¼ŹĒ·ÅČČ·“Ó¦ |
| D£®·“Ó¦Īļ×ÜÄÜĮæøßÓŚÉś³ÉĪļ×ÜÄÜĮ棬ŌņøĆ·“Ó¦Ņ»¶ØŹĒ·ÅČČ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹĒĪüČČ·“Ó¦ | B£®ŹĒ·ÅČČ·“Ó¦ |
| C£®ŹĒģŲ¼õŠ”µÄ·“Ó¦ | D£®ģŲŌöŠ§Ó¦“óÓŚČČŠ§Ó¦ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com