
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ������һ�ַ�Ӧԭ�����£�
CH3OH(g)��H2O(g)===CO2(g)��3H2(g)
��H��49.0 kJ·mol��1
����˵����ȷ���� (����)
A��1 L CH3OH������1 Lˮ������Ӧ����1 L CO2������3 L������������49.0 kJ
B��1��CH3OH������1��ˮ���ӷ�Ӧ����1��CO2������3��H2��������49.0 kJ����
C����ͬ������1 mol CH3OH(g)��1 mol H2O(g)�������ܺ�С��1 mol CO2(g)��3 mol H2(g)�������ܺ�
D��1 mol CH3OH������1 molҺ̬ˮ��Ӧ����1 mol CO2������3 mol�������յ�����С��49.0 kJ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪C3N4�����Ӳ�Ⱥܿ��ܱȽ��ʯ������ԭ�Ӽ���Ե�����ϣ����й���C3N4��˵����ȷ����
A��C3N4�����Ƿ��Ӿ���
B��C3N4������C��N���ļ����Ƚ��ʯ��C��C���ļ���Ҫ��
C��C3N4������ÿ��Cԭ������4��Nԭ�ӣ�ÿ��Nԭ������3��Cԭ��
D���þ�������ʯ���ƣ�����ԭ�Ӽ��ԷǼ��Լ��γɿռ����״�ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���ȷ�Ӧ����Ҫ���Ⱦ��ܷ�Ӧ�����ȷ�Ӧ�����ȾͲ��ܷ�Ӧ (����)
(2)���ʷ�����ѧ�仯�����������ı仯 (����)
(3)���������仯�����ʱ仯���ǻ�ѧ�仯 (����)
(4)���ȷ�Ӧ���κ����������ܷ��� (����)
(5)Naת��ΪNa��ʱ�����յ��������Ǹù��̵ķ�Ӧ�� (����)
(6)ˮ������ΪҺ̬ˮʱ�ų����������Ǹñ仯�ķ�Ӧ�� (����)
(7)ͬ��ͬѹ�£���ӦH2(g)��Cl2(g)===2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ(����)
(8)���淴Ӧ�Ħ�H��ʾ��ȫ��Ӧʱ�������仯���뷴Ӧ�Ƿ������ (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�̫���ܵ����÷�ʽ�Լ��оٵ�ʵ��������� (����)
A��ֱ������̫�������ܵĻ�����ʽ�����֣���—��ת������—��ת������—��ѧ��ת����—��������ת��
B��̫������ˮ����һ�ֹ�—��ת����ʽ
C����ɫֲ����еĹ�����ã���һ�ֹ�—��������ת�������ı����ǹ�—��ѧ��ת��
D��������ͨ���õ������������Ĺ�����һ�ֹ�—����ת������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������С�⡣
(1)[2013·���գ�20(1)]����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)��10C(s)===6CaO(s)��P4(s)��10CO(g)����H1����3 359.26 kJ·mol��1
CaO(s)��SiO2(s)===CaSiO3(s)����H2����89.61 kJ·mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)===6CaSiO3(s)��P4(s)��10CO(g)����H3
��H3��________kJ·mol��1��
(2)[2013·�Ĵ����ۣ�11(5)��ѡ]���ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)��O2(g)2SO3(g)����H1����197 kJ·mol��1��
H2O(g)===H2O(l)����H2����44 kJ·mol��1��
2SO2(g)��O2(g)��2H2O(g)===2H2SO4(l)
��H3����545 kJ·mol��1��
��SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��________��
(3)[2013·�㽭���ۣ�27(1)]��̼����(��Ҫָ����CO2)�ڽ������������ŷ��о�����Ҫ�����á�ĿǰNH3��(NH4)2CO3�Ѿ���������ҵ��̼����������CO2�ɷ������¿��淴Ӧ��
��Ӧ��2NH3(l)��H2O(l)��CO2(g)(NH4)2CO3(aq)��H1
��Ӧ��NH3(l)��H2O(l)��CO2(g)NH4HCO3(aq)��H2
��Ӧ��(NH4)2CO3(aq)��H2O(l)��CO2(g)2NH4HCO3(aq)��H3
��H3�릤H1����H2֮��Ĺ�ϵ�ǣ���H3��________��
(4)[2013·������ۣ�10(2)��]Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
��úת��Ϊ�������ȼ�ϡ�
��֪��H2(g)�� O2(g)===H2O(g)
O2(g)===H2O(g)
��H����241.8 kJ·mol��1
C(s)�� O2(g)===CO(g)����H����110.5 kJ·mol��1
O2(g)===CO(g)����H����110.5 kJ·mol��1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ��____________________________________��
(5)[2013·�������ۣ�26(2)��]��������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�
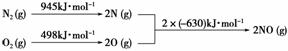
д���÷�Ӧ���Ȼ�ѧ����ʽ��_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������£�0.01 mol·L��1 MOH��Һ��pHΪ10��MOH(aq)��H2SO4(aq)��Ӧ����1 mol���εĦ�H����24.2 kJ·mol��1��ǿ����ǿ���ϡ��Һ���к���Ϊ��H����57.3 kJ·mol��1����MOH��ˮ��Һ�е���Ħ�HΪ (����)
A����69.4 kJ·mol��1 B����45.2 kJ·mol��1
C��69.4 kJ·mol��1 D��45.2 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���³�ѹ�£�ȡ���������л����1mol���ֱ���������������ȼ�գ��������������ǣ� ��
A��C2H5OH B��CH4 C��C2H4O D��C3H8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ϩ��ʯ���ѽ�������Ҫ�ɷ֣�����ش��������⡣
(1)��ϩ�ĵ���ʽ____________���ṹ��ʽ____________��
(2)����������ϩ���Լ���______(�����)��
A��ϡ���� B��������Ȼ�̼��Һ
C��ˮ D�����Ը��������Һ
(3)���������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����______(�����)��
A��CH3CH3 B��CH3CHCl2
C��CH3CH2OH  D��CH3CH2Br
D��CH3CH2Br
(4)��֪ 2CH3CHO��O2 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
 |
��Ӧ�ڵĻ�ѧ����ʽΪ____________________________________��
��ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ____________________________________����Ӧ������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£��ס������ձ���ʢ��5mlpH��3��ijһԪ����Һ�������ձ��м�ˮϡ����pH��4�����ڼס������ձ�����Һ��������ȷ����
A����Һ�������10V����V��
B��ˮ�������OH��Ũ�ȣ�10c(OH��)����c(OH��)��
C�����ֱ��õ�Ũ�ȵ�NaOH��Һ��ȫ�кͣ�������Һ��pH���ס���
D�����ֱ���5mlpH��11��NaOH��Һ��Ӧ��������Һ��pH���ס���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com