



 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ�� | ��ʼ | 8h�� | 16h�� | 24h�� | 32h�� | 40h�� | 48h�� |
| pH | 5.0 | 4.8 | 4.6 | 4.3 | 4.2 | 4.0 | 4.0 |
�鿴�𰸺ͽ���>>
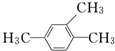
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
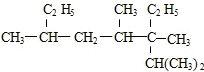
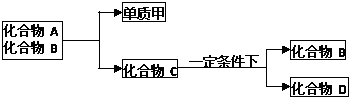
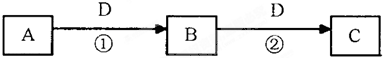
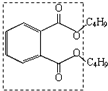 ���еĹ���������Ϊ
���еĹ���������Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
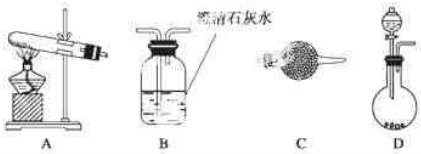
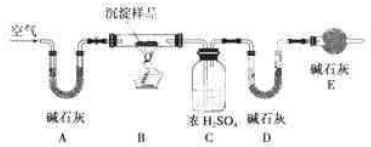
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ӦȡŨ��������/ml | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ú̿��������Һ������ȹ��̣�����ת��Ϊ�����Դ |
| B�����ö�����̼����ȫ�������ϣ����Ի�������ЧӦ |
| C����ֿ���������Ȼ��ά��ֹͣʹ�ø��ֻ�ѧ�ϳɲ��� |
| D���Ӵ�ոѵ��ۺ����ã��緢���������������Ҵ��ȣ������Դ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������KNO3��S����������Ԫ����C |
| B��ÿ����1molN2��ת�Ƶ��ӵ����ʵ���Ϊ12mol |
| C��ÿ����3.2gSʱ�����ɵ��������Ϊ8.96L |
| D����Ӧ�л�ԭ���뱻��ԭ���ʵ����ʵ���֮��Ϊ3��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com