
 �����£���0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ�������й�˵������ȷ���ǣ�������
�����£���0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ�������й�˵������ȷ���ǣ�������| A�� | a���pH��7����Kw��Ϊ1.0��10-14 | |
| B�� | b����ʾ��Һ��C��Cl-��=C��NH4+�� | |
| C�� | C����ҺpH��7����ԭ����NH4++H2O?NH3•H2O+H+ | |
| D�� | d����ʾ��Һ������Ũ���ɴ�С�������ǣ�c��Cl-����C��NH4+����C��H+����C��OH-�� |
���� A��Kwֻ���¶ȵ�Ӱ�죻
B����b�㣬��Һ��pH=7��
C����c����Һ�У��������������Ϊ20mL���ܽ���ˮǡ����ȫ�кͣ�
D����d�㣬�������������Ϊ40mL��������������õ���ҺΪ��Ũ�ȵ�HCl��NH4Cl�Ļ���
��� �⣺A��Kwֻ���¶ȵ�Ӱ�죬��a����Һ��Kw��Ϊ1.0��10-14����A��ȷ��
B����b�㣬��Һ��pH=7������C��H+��=C��OH-�������ݵ���غ��֪��C��Cl-��=C��NH4+������B��ȷ��
C����c����Һ�У��������������Ϊ20mL���ܽ���ˮǡ����ȫ�кͣ������õ���ҺΪNH4Cl��Һ����ҺpH��7����ԭ����NH4+����Һ�л�ˮ�⣺NH4++H2O?NH3•H2O+H+����C��ȷ��
D����d�㣬�������������Ϊ40mL��������������õ���ҺΪ��Ũ�ȵ�HCl��NH4Cl�Ļ�����Һ�����ԣ�������NH4+ˮ�⣬��NH4+��Ũ��С��H+Ũ�ȣ���ȷ��˳��Ϊ��c��Cl-����C��H+����C��NH4+����C��OH-������D����
��ѡD��
���� ���⿼��������Һ�ͼ���Һ��Ϲ��̵�����Ũ�ȴ�С�Ƚϡ���Һ��Kwֵ�ı仯ֻ���¶ȵ�Ӱ������⣬�ѶȲ���ע������Ũ�ȴ�С�Ƚϵ����գ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
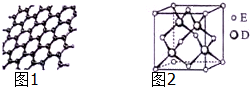 ԭ�������������������Ԫ��A��B��C��D��Eλ�����ڱ���ǰ�����ڣ�A��̬ԭ�ӵ�2p�������2��δ�ɶԵ��ӣ�C�������������Ǵ�����������3����C��Dͬ�������ڣ�Eλ�����ڱ���ds���������ֻ��һ�ԳɶԵ��ӣ���ش��������⣺
ԭ�������������������Ԫ��A��B��C��D��Eλ�����ڱ���ǰ�����ڣ�A��̬ԭ�ӵ�2p�������2��δ�ɶԵ��ӣ�C�������������Ǵ�����������3����C��Dͬ�������ڣ�Eλ�����ڱ���ds���������ֻ��һ�ԳɶԵ��ӣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��Cl-��Cu2+��Ba2+ | B�� | OH-��NO${\;}_{3}^{-}$��Ba2+��Cl- | ||
| C�� | H+��CO${\;}_{3}^{2-}$��Mg2+��Ba2+ | D�� | OH-��NO${\;}_{3}^{-}$��CO${\;}_{3}^{2-}$��Mg2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 1mol OH-���еĵ�����Ϊ9NA | |
| B�� | ���³�ѹ�£�NO2��N2O4�Ļ����23g�к���NA����ԭ�� | |
| C�� | ��״���£�2.8gN2��2.24L CO������������Ϊ1.4NA | |
| D�� | ��״���£�22.4 L �Ҵ��к���NA���Ҵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | ����Ũ�ȣ�mol•L-1�� | |
| ��ԭǰ | ��ԭ�� | |
| SO42- | 3.20 | 3.50 |
| Fe2+ | 0.15 | 3.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ֱ�������CaCO3��Ӧʱ���ų���CO2һ���� | |
| B�� | ��NaOH��ȫ�к�ʱ�����������ĵ�NaOH�� | |
| C�� | ������Һ��pH��� | |
| D�� | ȡ����������CH3COOH��Һ���ֱ��ˮϡ��a����b������Һ��pH��ȣ���a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ����Ba��OH��2��Һ | C�� | �ⶨ��Һ��pHֵ | D�� | ����Ʒ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��b+a�� kJ/mol | |
| B�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��2b+a��kJ/mol | |
| C�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��a-2b�� kJ/mol | |
| D�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��b-a�� kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com