
���� ��1����������ˮ���룬���������ӵ��δٽ�ˮ���룬����������Ũ��Խ����������������Ũ��Խ��������ˮ����̶�Խ��
��2����ѧʽ��笠����Ӹ�����ȵ�ǿ������У���������Ӵٽ�笠�����ˮ�⣬����������笠�����ˮ�⣬һˮ�ϰ���������ʣ������̶Ƚ�С��
��3����NH4Cl�͢�NH3•H2O�����Һ��
A�������Һ�����ԣ������Һ�е���غ�����жϣ�
B�����c��C1-��=c��NH4+����������Һ�е���غ�����жϣ�
C��������Һ�е���غ�����ж���Ũ�ȴ�С��
D����������Һ��������������Һ��������Һ�е������غ㣻
E��������Һ�϶��������غ㣻
��4��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룻��t��ʱ��pH=11����Һ��a L��pH=1����Һ��b L��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH=2����Һ�����ԣ���ϻ�Ϻ���Һ������Ũ����ʽ���㣻
��5�������£������Һ��CH3COONH4 ��pH=7��˵����Һ�����ԣ����������ˮ���Լ��ԣ�笠�����ˮ�������ԣ����̶߳���ͬ��
��� �⣺��1����������ˮ���룬���������ӵ��δٽ�ˮ���룬����������Ũ��Խ����������������Ũ��Խ��������ˮ����̶�Խ��Ũ�ȵ��⼸����Һ�У�NH4Cl�ٽ�ˮ���룬H2SO4��������Ũ����0.2mol/L��������������Ũ��С��0.1mol/L��NaOH������������Ũ��Ϊ0.1mol/L�����Ԣ٣��ڣ��ۣ���������Һ����ˮ�������H+Ũ���ɴ�С��˳���Ǣܣ��ڣ��ۣ��٣�
�ʴ�Ϊ���ܣ��ڣ��ۣ��٣�
��2����ѧʽ��笠����Ӹ�����ȵ�ǿ������У���������Ӵٽ�笠�����ˮ�⣬����������笠�����ˮ�⣬һˮ�ϰ���������ʣ������̶Ƚ�С�����Ե�Ũ�ȵ��⼸����Һ�У�笠�����Ũ�ȴ�С˳���ǣ��ޣ��ߣ��ܣ��ݣ��࣬
�ʴ�Ϊ���ޣ��ߣ��ܣ��ݣ��ࣻ
��3��A�������Һ�����ԣ�c��C1-��+c��OH-��=c��NH4+��+c��H+������c��C1-����c��NH4+����c��H+����c��OH-������A��ȷ��
B�����c��C1-��=c��NH4+��������غ�����c��C1-��+c��OH-��=c��NH4+��+c��H+����c��H+��=c��OH-��������Һ�����ԣ���B��ȷ��
C������غ�����c��C1-��+c��OH-��=c��NH4+��+c��H+����c��C1-����c��NH4+����c��OH-����c��H+������Ũ�ȴ�С������Ϊ��c��C1-����c��NH4+����c��OH-����c��H+������C����
D����������Һ��������������Һ�����ϵ��c��NH4+��+c��NH3•H2O��=2c��Cl-��=��0.1mol•L-1����D����
E��������Һ����غ�����c��C1-��+c��OH-��=c��NH4+��+c��H+�����϶������ϵ��c��C1-��+c��OH-��=c��NH4+��+c��H+������E��ȷ��
�ʴ�Ϊ��A B E��
��4��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ�����ӻ���������t��ʱ��Kw=1��10-13��10-14������t�棾25�棬
��t��ʱ��pH=11����Һ��a LNaOH ��Һ��pH=1����Һ��H2SO4��Һb L��ϣ����Ի�Ϻ���Һ����ı仯����NaOH ��Һ��c��OH-��=$\frac{1{0}^{-13}}{1{0}^{-11}}$=10-2mol/L��������Һ��c��H+��=0.1mol/L�������û����Һ��pH=2��$\frac{0.1mol/L��bL-0.01mol/L��aL}{aL+bL}$=0.01mol��l
a��b=9��2��
�ʴ�Ϊ������a��b=9��2��
��5�������£������Һ��CH3COONH4 ��pH=7��˵����Һ�����ԣ����������ˮ���Լ��ԣ�笠�����ˮ�������ԣ���˵��CH3COO-��ˮ��̶�=NH4+��ˮ��̶ȣ�
�ʴ�Ϊ��=��
���� ���⿼��������Ũ�ȴ�С���жϣ���ȷ������ʵĵ��뼰����ˮ���ص��ǽⱾ��ؼ�����ϵ����ǿ������Һ�е����ʽ������غ㡢����غ������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵ��Ĵ�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
�ں�����ߺ�DZˮͧ�п��ù���������Ϊ��������������ͼʵ��װ�ã�ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ����Ʋ���ɶԹ���������һ���ʵ�̽����
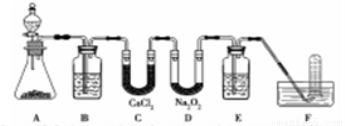
��1��A��ʵ������ȡCO2��װ�ã����з�����Ӧ�����ӷ���ʽ��___________________��װ��B��������____________��װ��C��������____________��װ��Eװ���Լ���___________��
��2������F������ɷ֣�����˵���������ƿ���DZˮͧ�������������������DZˮͧ�������Ļ�ѧ��Ӧ����Ϊ___________________________��
��3����Ʋ����ag����������Ʒ����֪������������������Ӧ���Ĵ��Ȳⶨ��������ˮ�ȼ�����У��ٰ�����ˮ�ֳ����ݣ�һ���г��ȼ�����Ʒ�ܽ⣬����ȴ�����º��ټ�����һ��ˮ���������ơ�ȡ�����Ƶ���Һ20.00mL�������̪���κ���Ũ��Ϊcmol/L��������еζ����ظ��ζ�����2�Σ������������������ƽ��ֵΪVmL��
�ٹ���������Ʒ�Ĵ��Ȳⶨ�����У����ȼ�����Ʒ�ܽ⣬������__________________��
��ԭ����������Ʒ�Ĵ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ������
��ͼ��Ԫ�����ڱ���һ����
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| B |
|
|
|
|
|
|
|
|
|
|
| C | D | E | F |
|
H |
|
|
|
|
|
|
|
|
|
|
| I |
| J |
| K | [Z |
|
|
|
| L |
|
|
| M |
|
|
|
|
|
|
| O |
|
��1��Ԫ��L�ļ۵����Ų�ͼΪ ��Ԫ��M��ԭ�ӽṹʾ��ͼΪ ��λ��Ԫ�����ڱ���������е� ��������Ԫ���е縺����ǿ���� ����Ԫ�ط��ű�ʾ����C��D��E��F��һ�����ܵĴ�С˳��Ϊ ����Ԫ�ط��ű�ʾ����
��2��D���⻯���J�⻯��е�ϸߵ��� ���ѧʽ�����ȶ��Ը�ǿ���� ���ѧʽ����
��3��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ��I��Ԫ��B���������������Ƶ����ʡ�д��Ԫ��B������������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��4��A��E��H��J����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С������˳��Ϊ ����Ԫ�ط��ű�ʾ����E��F��H��K����Ԫ�ؼ����ӵİ뾶�ɴ�С������˳��Ϊ ����Ԫ�ط��ű�ʾ����
��5����֤��Ԫ��K��Ԫ��J�ķǽ�����ǿ����ʵ�� ������ţ���
A��������K���ʵ��ܶ�С��J���ʵ��ܶ�
B��KԪ�ص���̬�⻯���JԪ�ص���̬�⻯����ȶ�
C��K������������Ӧ�ij̶ȱ�J������������Ӧ�ij̶ȸ�����
D��KԪ�ص��������Ӧ��ˮ��������ǿ��JԪ�ص��������Ӧ��ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ӦCO��g��+NO2��g��=CO2��g��+NO��g����H��0����ƽ��������¶���ϵ��ɫ���� | |
| B�� | ʵ�����Ʊ��������ô�п���洿п����Ӧ���ʼӿ� | |
| C�� | �����Ȼ�����Һʱ�����Ȼ������������У�Ȼ���ˮϡ�� | |
| D�� | �����£���1mLpH=3�Ĵ�����Һ��ˮϡ����l00mL�������pH��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ʵ���ɺ������ص㣬���Խ����ʷֳɻ����ʹ����Ư�ۡ��֡�ˮ������Һ����Ϊ����� | |
| B�� | �����ᡢ�����ʡ���֬�������ж�����C��H��O��N����Ԫ�� | |
| C�� | ���ϣ���������л������������̼����Ԫ�����������IJⶨ��ͬλ��ʾ�ٷ��������о���ѧ��Ӧ���̻��� | |
| D�� | ���ס������ֱ�����ˮ���γɵķ�ɢϵΪ���壬���ȡ���ȩ�����ͣ�NH4��2SO4��Һ��X���������£������ʵ����ʶ���ı䲢�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl ��H2SO4 | ��NaOH ��Ba��OH��2 | ��Na2CO3 ��K2SO4 | ��CO2 ��Na2O | ��NH3 ��H2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CH��CH ��HCl ��Ӧ������ϩ�������Ʊ�������ϩ���� | |
| B�� | Ũ��ˮ������ʯ���У�������������ͨ��AlCl3��Һ���ɵõ���AlO2-����Һ | |
| C�� | ʹ�ô������ܸı乤ҵ�ϳ�NH3�ķ�Ӧ�� | |
| D�� | �ý���KMnO4��Һ�Ĺ���������ˮ���ͷŵ���ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| ѡ�� | a | b | c |
| A | Fe | FeCl3 | FeCl2 |
| B | HCl | Cl2 | HClO |
| C | H2SO4 | SO2 | SO3 |
| D | CH2=CH2 | CH3CH2OH | CH3CHO |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com