
(18��)MnO2��Zn������ɵ�ص���Ҫԭ�ϣ���ҵ�������̿�(��Ҫ�ɷ�MnO2)����п��(��Ҫ�ɷ�ZnS)��������MnO2��Zn�Ĺ�����������ͼ��ʾ��
��1������I�õ������β��ʵ��������ձ�����������_______ _____(����������)��
��2��ϡ�������ʱ��Ӧ�����ӷ���ʽΪ_______________________________________���÷�Ӧ��������19.2g����A����ת��____________mo1���ӡ�����ʱ��Ӧ���ʽ��������д�ʩ������߽���ʱ��Ӧ���ʵ���________(�����)��
a������ʯ����
b����߽����¶�
c���ʵ���������Ũ��
d���ı����̿�����п��ı���
��3������������Һ�ɵõ�����̼���̣�Ȼ���ڿ���������̼�����Ʊ�MnO2����֪��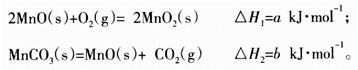
д��̼�����ڿ�������������MnO2���Ȼ�ѧ����ʽ_________________________��
��4���ö��Ե缫��������ữ����������Һ�Ʊ�MnO2��װ������ͼ��ʾ��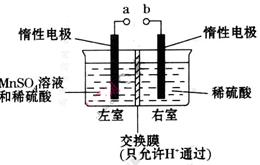
��aӦ��ֱ����Դ��_________(���������)����������
�ڵ������������ӵ�������______________��_____________����ת�Ƶĵ�����Ϊ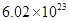 ����������Һ��
����������Һ��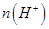 �ı仯��Ϊ________________��
�ı仯��Ϊ________________��
��1��©��
��2��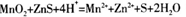 ��1.2��d
��1.2��d
��3��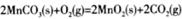 ��H=��a+2b��kJ/mol��
��H=��a+2b��kJ/mol��
��4�������ڲ���������Ӧ��ͨ������Ĥ�����ƶ��γɵ�����1mol
���������������1������IΪ���ˣ��õ������β��ʵ��������ձ�����������©������2��ϡ�������ʱ��ӦΪMnO2��ZnS��ΪMn2+��Zn2+��ͬʱӦ����S���ʣ��ʷ���ʽΪ��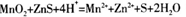 ��19.2g����S�����ʵ���Ϊ0.6mol���ɷ�Ӧ����ʽ��֪����1molSת��2mole-,������0.6molSת��1.2mole-���ĸ�ѡ���У�����ʯ���顢��߽����¶ȡ��ʵ���������Ũ�Ⱦ�����߷�Ӧ���ʣ��ʴ�Ϊd����3���ɸ�˹���ɽ���ӦI+II��2�ɵ�
��19.2g����S�����ʵ���Ϊ0.6mol���ɷ�Ӧ����ʽ��֪����1molSת��2mole-,������0.6molSת��1.2mole-���ĸ�ѡ���У�����ʯ���顢��߽����¶ȡ��ʵ���������Ũ�Ⱦ�����߷�Ӧ���ʣ��ʴ�Ϊd����3���ɸ�˹���ɽ���ӦI+II��2�ɵ�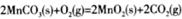 ��H=��a+2b��kJ/mol����4���ٸ���װ��ͼ��֪���ҷ���MnSO4��MnO2�ķ�Ӧ��������������Ӧ����aӦ��ֱ����Դ�������������ڵ�����������ΪH+�õ��ӷ�����ԭ��Ӧ���ֽ���Ĥֻ����H+ͨ������H+������Ϊ����������Ӧ��ͨ������Ĥ�����ƶ��γɵ�������ת�Ƶĵ�����Ϊ
��H=��a+2b��kJ/mol����4���ٸ���װ��ͼ��֪���ҷ���MnSO4��MnO2�ķ�Ӧ��������������Ӧ����aӦ��ֱ����Դ�������������ڵ�����������ΪH+�õ��ӷ�����ԭ��Ӧ���ֽ���Ĥֻ����H+ͨ������H+������Ϊ����������Ӧ��ͨ������Ĥ�����ƶ��γɵ�������ת�Ƶĵ�����Ϊ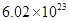 ����1mol�����ݵ���غ����������ʵ����仯Ϊ1mol��
����1mol�����ݵ���غ����������ʵ����仯Ϊ1mol��
���㣺���黯ѧʵ��������������ӷ���ʽ����д��������ԭ��Ӧ����ѧ��Ӧ����Ӱ�����ء���˹���ɡ��绯ѧ��֪ʶ��


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪298 K,101 kPaʱ��2SO2(g)��O2(g)  2SO3(g)����H����197 kJ��mol��1������ͬ�¶Ⱥ�ѹǿ�£����ܱ�������ͨ��2 mol SO2��1 mol O2���ﵽƽ��ʱ���ų�����ΪQ1������һ�������ͬ��������ͨ��1 mol SO2,0.5 mol O2��1 mol SO3���ﵽƽ��ʱ�ų�����ΪQ2�������й�ϵ��ȷ����(����)
2SO3(g)����H����197 kJ��mol��1������ͬ�¶Ⱥ�ѹǿ�£����ܱ�������ͨ��2 mol SO2��1 mol O2���ﵽƽ��ʱ���ų�����ΪQ1������һ�������ͬ��������ͨ��1 mol SO2,0.5 mol O2��1 mol SO3���ﵽƽ��ʱ�ų�����ΪQ2�������й�ϵ��ȷ����(����)
| A��Q2��Q1��197 kJ��mol��1 | B��Q2��Q1��197 kJ��mol��1 |
| C��Q1��Q2��197 kJ��mol��1 | D��Q2��Q1��197 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��3�֣���ѧ��һֱ�����ڡ��˹��̵��� �ķ����о���Ŀǰ�ϳɰ��ļ���ԭ��Ϊ�����������ڸ��¸�ѹ�������������ɰ�����һ�������£���һ��1L���ܱ������г���2molN2��6molH2����Ӧ��ƽ��ʱ����NH3��Ũ��Ϊ1 mol��L-1�����ų�Q kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪ ____ __��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣����о���ѧ��Ӧ�е������仯ʱ������ͨ���������ʵ�飺
��һ��С�ձ������20 g����ĥ�ɷ�ĩ��Ba(OH)2��8H2O����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��м���Լ10 g NH4Cl���壬����ʵ�鲽�裬��д�±������ش����⡣
| ʵ�鲽�� | ʵ�������� |
| �������ϣ��������ٽ��� | �д̼�����ζ��ʹʪ�����ɫʯ����ֽ������___��__���� |
| �������ձ��²� | �о��ձ�����˵���˷�Ӧ��_ �� ��Ӧ |
| ���������ձ� | �ձ�����Ĵ��м���ˮ�IJ���Ƭճ�����ձ��ײ� |
| ��ճ�в���Ƭ���ձ�����ʢ����ˮ���ձ��� | ����Ƭ���������ձ��ײ� |
| ��Ӧ�����߶������Ƭ�۲췴Ӧ�� | �����ɺ�״��֤����_��___���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣���Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij��ѧ�о�С���������̿���Ҫ�ɷ�ΪMnO2������������ͷ��������ͭ�����Ƚ�����������������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2����Ӧ��������ȥ����
��ش��������⣺
��1��������������ʵ����____��ѡ��������ĸ��ţ���
A����������ۺ����� B����ɫ��Ⱦ�ļ��� C������ļ���
��2����MnCO3�ܳ�ȥ��Һ��Al3����Fe3������ԭ����_____��
��3����֪��25�桢101kpaʱ��Mn(s)��O2(g)��MnO2(s) ��H����520kJ/mol
S(s)��O2(g)��SO2(g) ��H����297kJ/mol
Mn(s)��S(s)��2O2(g)��MnSO4(s) ��H����1065kJ/mol
SO2��MnO2��Ӧ������ˮMnSO4���Ȼ�ѧ����ʽ��________________��
��4��MnO2�����������������ϡ��ö��Ե缫���MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ��
_ _��
��5��MnO2�Ǽ���п�̵�ص��������ϡ�����п�̵�طŵ�ʱ�������ĵ缫��Ӧʽ��______��
��6�������ѳ���SO2ֻ�����̿��е�MnO2��Ӧ������ͼʾ���̣���a m3����״������SO2���������Ϊb%��β��ͨ�����SO2���ѳ���Ϊ89.6%�����յõ�MnO2������Ϊc kg�����ȥ��������ͭ����������ʱ�����������Ԫ���൱��MnO2___________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣�
I����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2SO2��g��ʮO2��g�� 2SO3��g��
2SO3��g��
��1���˷�Ӧ�ǹ�ҵ������ ����Ҫ���衣
��2���ܹ�˵���ں��º��������£�������Ӧ�Ѿ��ﵽ��ѧƽ��״̬���� ������ţ���
a��ÿ����1mol SO3��ͬʱ����0.5mol O2
b�������л��������ܶȲ���
c��SO2��O2��SO3�����ʵ���֮��Ϊ2��1��2
d��������������ѹǿ����
��3����400��ʱ����ѹ�£��ݻ�Ϊ1.0L���ܱ������г���1.00mol SO2(g)��0.96mol O2(g)����ַ�Ӧ��û���0.04mol SO2ʣ�࣬���ų�190.08KJ��������
�ٸ����й����ݣ�������ڹ�ҵ������ѡ��ѹ��Ӧ����ԭ��
��
��д���˷�Ӧ���Ȼ�ѧ����ʽ��
2SO2��g��ʮO2��g�� 2SO3��g�� ��H�� ��
2SO3��g�� ��H�� ��
��1����ij�¶��£���1.00 mol NH3����ˮ�����1.00 L��Һ�������Һ��OH��Ũ�Ⱥ�ʱ���ͼ�����£�
������¶�ʱ����ˮ�ĵ���ƽ�ⳣ��K�� ��
�� ��t1ʱ���ټ���H2O���2L��Һ����t2ʱ�����´ﵽƽ�⣬��������ϵ�л���t1��t2ʱ����OH��Ũ����ʱ��仯�����ߡ�
��2����a mol/L�������b mol/L��ˮ�������ϣ���Ϻ����Ϊ���ǰ���֮�ͣ���ַ�Ӧ��������Һ�����ԡ�
�� a b ���<������=����>����
�� ���������غ�ԭ�������Ϻ���Һ��ʣ�ఱˮŨ�ȣ�c(NH3��H2O)�� ��
���ú���a��b��ʽ�ӱ�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣������ѣ�CH3OCH3���ͼ״���CH3OH�����Ǹ�Ч�����Դ����ҵ������ú���������ˮú�����ϳɼ״��Ͷ����ѡ��ش��������⣺
��1���Ʊ����������һ����Ӧ��Al2O3���״���ˮ�ϳɣ���Ӧ����ʽΪ ��
��2����֪��CO(g)+2H2(g)=CH3OH (g) ��H= ��90.1kJ��mol-1 CO(g)��ȼ������282.8 kJ��mol-1��H2��ȼ������285.8 kJ��mol-1д����ʾCH3OH (g) ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ ��
��3��������ֱ��ȼ�ϵ�رȼ״�ֱ��ȼ�ϵ�ظ���Ч���������Ķ����Ѻͼ״���ȫ�ŵ�ת�Ƶ��ӵ����ʵ���֮���� ���ö�����ֱ��ȼ�ϵ�ص����������ʳ��ˮ��������9.2g������ʱ������������������������Ϊ L��������£�
��4���ںϳ��а���ˮú��������Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
| �¶� | 260�� | 280�� | 295�� | 310�� |
| COת���� | 89% | 80% | 75% | 60% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ǵ�ѭ���е���Ҫ���ʣ�����������������������Ź㷺��Ӧ�á�
��1����ͼ��N2��H2��Ӧ�����������仯��ʾ��ͼ����÷�Ӧ����Һ̬�����Ȼ�ѧ����ʽ�� ��
��2����֪����H2O(g)=H2O(l) ��H����Q1 kJ��mol��1
��C2H5OH(g)��3O2(g)=2CO2(g)��3H2O(g) ��H����Q2 kJ/mol ��C2H5OH(g)=C2H5OH(l) ��H����Q3 kJ/mol
��23 gҺ��ƾ���ȫȼ������CO2(g)��H2O(l)���ͷų�������Ϊ kJ������Q1��Q2��Q3����ʾ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ù��ܺ�������ɽ� CO2�� H2O(g)ת��Ϊ CH4�� O2�����������ʱ���ڲ�ͬ����(��)�����£�CH4���������ʱ��ı仯��ͼ��ʾ��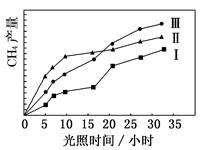
(1)��0��30 Сʱ�ڣ�CH4��ƽ���������� v����v����v���Ӵ�С��˳��Ϊ________����Ӧ��ʼ��� 12 Сʱ�ڣ��ڵ�________�ִ��������£��ռ��� CH4��ࡣ
(2)������ CH4�� H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ��CH4(g)��H2O(g)  CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1��
CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1��
�ٻ�����Ӧ��������ϵ�����仯ͼ(���б�Ҫ��ע)��
�ڽ������ʵ�����CH4�� H2O(g)���� 1 L �����ܱշ�Ӧ���У�ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ�� K �� 27����ʱ��� CO �����ʵ���Ϊ 0.10 mol����CH4��ƽ��ת����(������������λ��Ч����)��
(3)��֪��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����802 kJ��mol��1��
д���� CO2���� CO ���Ȼ�ѧ����ʽ____________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com