
��10�֣��������ԭ�����ش����и�С�⣺
��1��25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c(OH-)= �������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ�� ��
��2����֪NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4��Na+��H+��SO42-��
��NaHSO4��Һ��c(H+) c(OH-)��c(SO42-)���>������������<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ����Һ��pH 7��
��3����0.02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ��(��֪Ksp(BaSO4)��1.1��10-10)
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
����������Ӧ �� �Ҵ��Ĵ�������Ӧ ��
��10�֣�
��1��10-2mol/L ��1�֣� Fe3++3H2O Fe(OH)3(����)+3H+ ��1�֣�
Fe(OH)3(����)+3H+ ��1�֣�
��2�� =��1�֣� >��1�֣�
��3�� 2.2��10-8mol/L��2�֣� ��4�� 2 C2H5OH + O2
2 C2H5OH + O2  2 CH3CHO + 2H2O����2�֣�
2 CH3CHO + 2H2O����2�֣�
���������������1��FeCl3��ǿ�������Σ�����ˮ����ˮ������ԣ���Fe3++3H2O Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L��
Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L��
���㣺����Ũ�ȵļ����Լ���С�ıȽ� ��ѧ����ʽ����д �ܽ�ƽ��
����������֪ʶ��Ƚϴ�˼������������Һ�У�������Ũ�ȳ������������ӵ�Ũ�ȵ���ˮ�����ӻ�����������ˮ��������������ӵ�Ũ�ȵ�����ˮ����������ӵ�Ũ�ȡ�NaHSO4��ˮ������ȫ���롣Ksp�ļ��㣬��������AnBm��s��="n" Am+(aq)+ mBn-(aq), �ܶȻ�(Ksp)=(C(Am+) )^n ( C(Bn-))^m��


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | ���볣�� |
| HClO | Ka=3��10-8 |
| H2CO3 | Ka1=4.3��10-7 |
| Ka2=5.6��10-11 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣��������ԭ�����ش����и�С�⣺
��֪��![]() ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ![]()
(1)�����£�PH=5��![]() ��Һ��ˮ�ĵ���̶� PH=9��
��Һ��ˮ�ĵ���̶� PH=9��![]() ��ˮ�ĵ���̶ȡ����>������=����<����
��ˮ�ĵ���̶ȡ����>������=����<����
��2������������ʵ���Ũ�ȵ�![]() �백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������
�백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������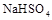 ��Һ�백ˮ��Ϻ���ҺPH=7����
��Һ�백ˮ��Ϻ���ҺPH=7����
![]()
![]() ���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��
���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��![]() ��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
(3)���ֱ���![]() ��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
����![]() ������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
�������û��Һ����ɫ������������������Һ��һ�����ڵ��� ��
һ�������ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�����е�һ��ѧ����12�½��Բ��Ի�ѧ�Ծ� ���ͣ������
�������ԭ�����ش����и�С�⣺
��֪��NaHSO4��ˮ�еĵ��뷽��ʽΪ NaHSO4��Na�� + H�� + SO42��
��1�������£�pH=5��NaHSO4��Һ��ˮ�ĵ���̶� pH=9��NH3��H2O��ˮ�ĵ���̶ȡ����� ��������������������
��2������������ʵ���Ũ�ȵ�NaHSO4�백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������NaHSO4��Һ�백ˮ��Ϻ���ҺpH=7����[Na��]��[NH4��] 2[SO42��]���� ��������������������������������������������Һ��ȡ���ᱵ������Һ��SO42����ȫ��������Ӧ����Һ��pH 7��������������������� ��
��3�����ֱ���MnO4����Fe3+��Fe2+��I����������Һ��ϣ�������Һ��pHֵ��ʹpH=1��
��ַ�Ӧ��
����I��������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ��� ��
�������û��Һ����ɫ������������������Һ��һ�����ڵ��� ��
һ�������ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и�����ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
��14�֣��������ԭ�����ش����и�С�⣺
��֪��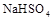 ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ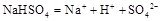
(1)�����£�PH=5��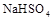 ��Һ��ˮ�ĵ���̶� PH=9��
��Һ��ˮ�ĵ���̶� PH=9��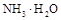 ��ˮ�ĵ���̶ȡ����>������=����<����
��ˮ�ĵ���̶ȡ����>������=����<����
��2������������ʵ���Ũ�ȵ�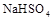 �백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������
�백ˮ��Ϻ���Һ�����Ե�ԭ��Ϊ ���������ӷ���ʽ��ʾ������һ������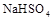 ��Һ�백ˮ��Ϻ���ҺPH=7����
��Һ�백ˮ��Ϻ���ҺPH=7����
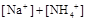
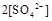 ���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��
���>������=����<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��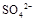 ��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
��ȫ��������Ӧ����Һ��PH= 7���>������=����<����
(3)���ֱ���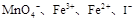 ��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
��������Һ��ϣ�������Һ��PHֵ��ʹPH=1����ַ�Ӧ��
����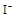 ������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
������ʣ�࣬����������������Һ�л����ڵ��� ��һ�������ڵ���
�������û��Һ����ɫ������������������Һ��һ�����ڵ��� ��
һ�������ڵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com