
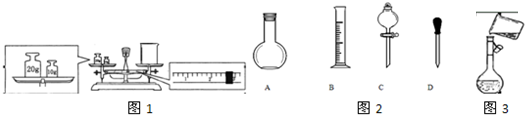
��12�֣� ijͬѧ��ʵ�������������ʵ���Ũ�Ⱦ�Ϊ1��0 mol/L��NaOH��Һ��ϡH2SO4��450mL���ṩ���Լ��ǣ�NaOH�����98%��ŨH2SO4���ܶ�Ϊ1��84 g/cm3��������ˮ��
��1������������Һʱ����Ҫ�IJ���������____________________________________ ��
��2��Ӧ��������ƽ����NaOH ___________g��Ӧ����Ͳ��ȡŨH2SO4________mL��
��3������ʱ����Ҫ�������ƿ�Ƿ�©ˮ���䷽����
��
��4��Ũ��������ˮ����ȷ����������_____ ___________��
��5��������������Һʵ���У����в���������ƫ�͵���_________________
A����ѧ������ȡŨ����ʱ�����ӿ̶���
B����������NaOHʱ�����������Ʒ��λ�õߵ���û��ʹ�����룩
C���ܽ�H2SO4����ʱû����ȴ�����¾�������ɺ�������Ʋ�����
D�����ձ����ܽ����ʱ������������Һ
E��û��������ˮϴ�ձ�2��3�Σ�����ϴҺ��������ƿ��
F������Ͳϴ��2��3�Σ���ȫ��ת��������ƿ��
G������ƿ��ԭ��������������ˮ
H����ͷ�ιܼ�ˮ����ʱ���ӿ̶�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������ƽ�ϳ�ȡԼ1.7��Na2CO3���� | B��ϴ���ձ������Һ�ò���������������ƿ�� | C��ϴ��2-3�κ�������ˮ�ز�����ע������ƿ���̶��� | D��ijͬѧ�������ӵķ�ʽ���ݣ����������ҺŨ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
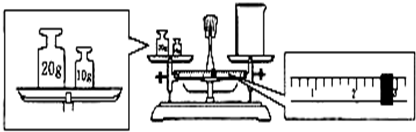
(14��)��������׳�PP�ۣ�Ϊǿ�������������л���ͷų�����������������ɱ��ϸ�������ã���ɱ��������ǿ��ijѧ������ʵ��������1 L 0.06 mol/L KMnO4ϡ��Һ��������ϴ�˿ڡ�
(1)ʵ�����������������ƽ��ȡKMnO4���������Ϊ__________g��
(2)�������������ͼ��ʾ������ͼ��ʾ����Ӧ����ͼ��__________(��ѡ����ĸ)֮�䡣
A������ۡ���B������ڡ���C�������
(3) ���ƹ�������Ҫ�õ���������:������ƽ��ҩ�ס��ձ��� �� ��
(4)����ͬѧ��������Һʱ�����������²���������ʹ������ҺŨ��ƫ�͵IJ���
��_______________(��ѡ����ĸ)��
A������KMnO4����ʱ��ָ��ƫ���ұ�
B����KMnO4��������ձ����ܽ⣬��ȴ��ת����������������ˮ������ƿ��
C������ʱ�����ӿ̶���
D����ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ٵμ���������ˮ���̶��ߴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��19�֣�����������̵���Ҫ������ͳ��õ���������������ʵ������ģ�ҵ�������̿��Ʊ�������ص�����ͼ��
��1�������������Ϊ �������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ����ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe��������K2MnO4Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2����MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԣ� ��
��4��KMnO4�����Խ����е�ǿ�����Թ㷺Ӧ���ڷ�����ѧ�С�
���磺2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O��ijͬѧ��KMnO4�ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȡ�����ȷ��ȡ6.3 gNa2SO3������Ʒ�����500 mL��Һ��ȡ25.00 mL������Һ������ƿ�У���0.01000 mol/L ������KMnO4��Һ���еζ����ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 mL | 0.02 | 24.01 |
| 2 | 25.00 mL | 0.70 | 24.71 |
| 3 | 25.00 mL | 0.20 | 24.20 |
������500 mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ�������������ͷ�ιܡ�ҩ�� �� ��
���жϵζ��յ�������� ��
�����в����ᵼ�²ⶨ���ƫ�ߵ���
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿδ����
C���ζ�ǰ�ζ��ܼ��첿��������
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��������ʵ�����ݣ�����Na2SO3�Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com