ЈЁ2013?єјЦЭТ»ДЈЈ©ПВ±нОЄФЄЛШЦЬЖЪ±нµДТ»Ії·ЦЈ¬ЗлІОХХФЄЛШўЩЎ«ўаФЪ±нЦРµДО»ЦГЈ¬»ШґрПВБРОКМвЈ»ЈЁ»ШґрОКМвК±Ј¬ѕщРиК№УГФЄЛШµДХжКµФЄЛШ·ыєЕЈ¬І»µГК№УГКэЧЦ±аєЕЈ©
Че
ЦЬЖЪ |
ўсA |
ўтA |
ўуA |
ўфA |
ўхA |
ўцA |
ўчA |
ўш |
| 1 |
ўЩ |
|
|
|
|
|
|
|
| 2 |
|
|
|
ўЪ |
ўЫ |
ўЬ |
|
|
| 3 |
ўЭ |
|
ўЮ |
ўЯ |
|
|
ўа |
|
ЈЁ1Ј©ўЪЎўўЭЎўўЮµДВГЧУ°лѕ¶УЙґуµЅРЎµДЛіРтОЄ
NaЈѕAlЈѕC
NaЈѕAlЈѕC
ЈЁ2Ј©РґіцУЙўЩЎўўЪБЅФЄіЖЧйіЙЈ¬ЗТўЩµДЦКБї·ЦКэЧоёЯµД·ЦЧУµДµзЧУКЅ
Ј¬
РґіцёГ»ЇєПОпёъўаµДµҐЦК·ўЙъЦГ»»·ґУ¦µД»ЇС§·ЅіМКЅ
Ј®
ЈЁ3Ј©ФЄЛШўЮµДЧоёЯјЫСх»ЇОпїЙєНФЄЛШўЭµДЗвСх»ЇОпЛ®ИЬТє·ўЙъ·ґУ¦Ј¬Рґіц·ґУ¦µДАлЧУ·ЅіМКЅ
Al2O3+2OH-ЁT2AlO2-+H2O
Al2O3+2OH-ЁT2AlO2-+H2O
ЈЁ4Ј©ФЄЛШўЫУР¶аЦЦСх»ЇОпЈ¬ЖдЦРјЧµДПа¶Ф·ЦЧУЦКБїЧоРЎЈ»ФЄЛШўЬУР¶аЦЦµҐЦКЈ¬ЖдЦРТТµДПа¶Ф·ЦЧУЦКБїЧоРЎЈ®ФЪТ»¶ЁМхјюПВЈ¬Ѕ«2L јЧЖшМеУл1.5L ТТЖшМеѕщФИ»мєПЈ¬ИфёГ»мєПЖшМе±»ЧгБїNaOH ИЬТєНкИ«ОьКХЈЁГ»УРЖшМеІРБфЈ©Ј®ЛщЙъіЙµДє¬СхЛбСОµД»ЇС§КЅКЗ
NaNO3
NaNO3
Ј®

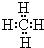

 Ј¬
Ј¬ Ј»CH4+2Cl2
Ј»CH4+2Cl2

 МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё
МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
 ЈЁ2013?єјЦЭТ»ДЈЈ©ИзНј±нКѕЖыіµОІЖшѕ»»ЇЖчґ¦АнЖыіµОІЖшµД№эіМЈ®УР№ШРрКцХэИ·µДКЗЈЁЎЎЎЎЈ©
ЈЁ2013?єјЦЭТ»ДЈЈ©ИзНј±нКѕЖыіµОІЖшѕ»»ЇЖчґ¦АнЖыіµОІЖшµД№эіМЈ®УР№ШРрКцХэИ·µДКЗЈЁЎЎЎЎЈ© ЈЁ2013?єјЦЭТ»ДЈЈ©АыУГіЈОВПВ°±УлВИЖшДЬ·ўЙъЦГ»»·ґУ¦µДРФЦКЈ¬ВȼҵЙъІъЦРУГАґјмІйВИЖшКЗ·сР№В©Ј¬ЖдДЈДвЧ°ЦГИзНјЈ®ПВБРУР№ШЛµ·ЁґнОуµДКЗЈЁЎЎЎЎЈ©
ЈЁ2013?єјЦЭТ»ДЈЈ©АыУГіЈОВПВ°±УлВИЖшДЬ·ўЙъЦГ»»·ґУ¦µДРФЦКЈ¬ВȼҵЙъІъЦРУГАґјмІйВИЖшКЗ·сР№В©Ј¬ЖдДЈДвЧ°ЦГИзНјЈ®ПВБРУР№ШЛµ·ЁґнОуµДКЗЈЁЎЎЎЎЈ©