
| A������NaOH��Һ�����ȣ�����ʹʪ�����ɫʯ����ֽ�������壬��ԭ��Һ��һ����NH4+ |
| B������������CaCl2��Һ�������˰�ɫ��������Һ��һ���д�����CO32�� |
| C���ýྻ�IJ�˿պȡ������Һ�����ڻ��������գ�����ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܺ��������� |
| D���ȼ����������Ὣ��Һ�ữ���ټ�AgNO3��Һ�������˰�ɫ��������Һ��һ�����д�����Cl�� |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
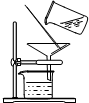
| A�����ͺ�ֲ���� | B��̼��ƺ�ˮ |
| C���ƾ���ˮ | D�����Ȼ�̼�͵� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3COOH��CH3CH2OH | B��Al(OH)3��ˮ |
C��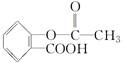 �ͱ� �ͱ� | D��CH3CH2OH��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
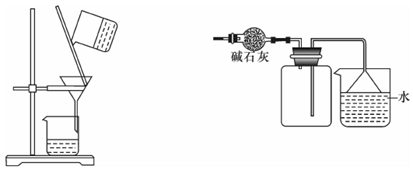

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
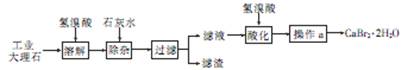
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
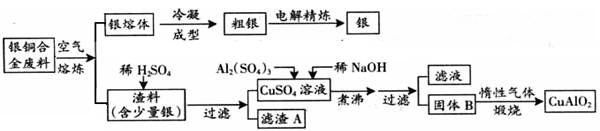
 Cu AlO2 �� ��ϵ��1ҲҪд��.
Cu AlO2 �� ��ϵ��1ҲҪд��.�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
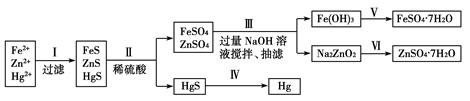
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ᴿNaCl��KNO3�Ļ�����е�KNO3��������������ȴ�ᾧ������ |
| B����ȥCO2�л��е�����CO�������������ͨ��NaOH��Һ��Ũ���� |
| C����ȥ�����л��е�����CaCl2���������CaCO3��ĩ������ |
| D����ȥNaCl��Һ�л��е�����I2��������Һ�м�������CCl4���������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KNO3��Һ(K2SO4)������Ba(NO3)2��Һ |
| B��CaCO3��ĩ(CaCl2)���������� |
| C��Cu��(Zn��)���������� |
| D��CO2(O2)�����ȵ�ͭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com