
2012Ρξ12‘¬Θ§Έ“Ιζ≥…ΙΠ Βœ÷ΦΏΓΣ15Ζ…Μζ‘ΎΓΑΝ…ΡΰΚ≈Γ±ΚΫΡΗ…œΒΡΤπΫΒ Β―ιΘ§Ϋ®‘λΚΫΡΗΤΫΧ®ΚΆΫΔ‘ΊΖ…Μζάκ≤ΜΩΣ»γν―ΓΔΈο÷ AΒ»≤ΡΝœΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)–¥≥ων―ΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΘΚ___________________________ΓΘ
(2)“―÷Σ‘ΣΥΊA¥Π”ΎΒΎ3÷ήΤΎΘ§Τδ‘≠Ή”ΒΡΒΎ“Μ÷ΝΒΎΥΡΒγάκΡή ΐΨί»γ±μΥυ ΨΘΚ
ΒγάκΡή(kJ/mol) | I1 | I2 | I3 | I4 |
A | 738 | 1 451 | 7 733 | 10 540 |
ΔΌAΒΡ‘ΣΥΊΖϊΚ≈ΈΣ________ΓΘ
ΔΎ”κ‘ΣΥΊAΆ§÷ήΤΎΒΡΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊΈΣ________(Χν‘ΣΥΊΖϊΚ≈)Θ§ΗΟ‘ΣΥΊΡή”κΆ§÷ήΤΎΒΡΝμ“Μ÷÷Ζ«Ϋπ τ‘ΣΥΊ–Έ≥…5‘≠Ή”Ζ÷Ή”Θ§ΗΟΖ÷Ή”ΒΡ÷––Ρ‘≠Ή”≤…”ΟΒΡ‘”Μ·ΖΫ Ϋ «________Θ§ΗΟΖ÷Ή”ΒΡΩ’ΦδΙΙ–ΆΈΣ________ΓΘ
(3)ν―ΟΨΚœΫπ «÷Τ‘λΗΏ–‘ΡήΖ…ΜζΒΡ÷Ί“Σ≤ΡΝœΓΘ“―÷Σν―ΓΔΟΨΫπ τΨυ≤…”ΟΝυΖΫΉνΟήΕ―Μΐ( )Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________(Χν–ρΚ≈)ΓΘ
)Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________(Χν–ρΚ≈)ΓΘ
AΘ°”Ον―ΟΨΚœΫπά¥÷Τ‘λΗΏ–‘ΡήΖ…ΜζΘ§÷ς“Σ”…”ΎΤδΦέΗώΑΚΙσΘ§÷Τ‘λ≥ωΒΡΖ…ΜζΡή¬τΗωΚΟΦέ«°
BΘ°‘ΎΗΏΈ¬œ¬«–ΗνΫπ τν―≤ΜΜα≤ζ…ζ»ΈΚΈΑ≤»Ϊ ¬Ι
CΘ°ν―ΓΔΟΨΫπ τΨßΧε÷–Θ§Τδ≈δΈΜ ΐΨυΈΣ12
DΘ°Ϋπ τν―ΒΡ»έΒψΚήΗΏ(1 668 Γφ)Θ§”κΫπ τΦϋΙΊœΒ≤Μ¥σ
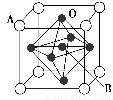
(4)ΗΤν―–ΆΗ¥Κœ―θΜ·Έο(»γΆΦΥυ Ψ)Ω…”Ο”Ύ÷Τ‘λΚΫΩ’ΡΗΫΔ÷–ΒΡ»»Οτ¥ΪΗ–ΤςΘ§ΗΟΨßΧε“‘A‘≠Ή”ΈΣΨßΑϊΒΡΕΞΒψΘ§AΩ…“‘ «CaΓΔSrΓΔBaΜρPbΘ§Β±B «VΓΔCrΓΔMnΜρFe ±Θ§’β÷÷Μ·ΚœΈοΨΏ”–ΚήΚΟΒΡΒγΜ·―ß–‘ΡήΓΘ”ΟAΓΔBΓΔO±μ ΨΗΤν―–ΆΗ¥Κœ―θΜ·ΈοΨßΧεΒΡΜ·―ß ΫΈΣ________ΓΘ
ΓΓ(1)1s22s22p63s23p63d24s2(Μρ[Ar]3d24s2)
(2)ΔΌMgΓΓΔΎClΓΓsp3‘”Μ·ΓΓ’ΐΥΡΟφΧε
(3)CΓΓ(4)ABO3
ΓΨΫβΈωΓΩΓΓ(1)ν―ΒΡ‘≠Ή”–ρ ΐΈΣ22Θ§ΤδΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d24s2ΓΘ(2)ΔΌ”…‘ΣΥΊAΒΡΒγάκΡή ΐΨίΩ…÷ΣΘ§ΒΎ“ΜΚΆΒΎΕΰΒγάκΡήœύΕ‘Ϋœ–ΓΘ§ΒΎ»ΐΚΆΒΎΥΡΒγάκΡήœύΕ‘Ϋœ¥σΘ§Ι ‘ΣΥΊA”ΠΈΣΒΎ3÷ήΤΎΔρAΉεΒΡMgΓΘΔΎ”κMgΆ§÷ήΤΎΒΡΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊΈΣClΘ§”κClΆ§÷ήΤΎΒΡΖ«Ϋπ τ‘ΣΥΊΜΙ”–SiΓΔPΓΔSΘ§ΒΪΡή”κCl–Έ≥…5‘≠Ή”Ζ÷Ή”ΒΡ‘ΣΥΊΈΣSiΘ§SiCl4÷–Si‘≠Ή”≤…”Οsp3‘”Μ·Θ§ΗΟΖ÷Ή”ΒΡΩ’ΦδΙΙ–ΆΈΣ’ΐΥΡΟφΧεΓΘ(3)―ΓœνAΘ§”Ον―ΟΨΚœΫπά¥÷Τ‘λΗΏ–‘ΡήΖ…Μζ”κ≤ΡΝœΒΡΫαΙΙΚΆ–‘÷ ”–ΙΊΘ§≤Δ≤Μ‘Ύ”ΎΤδΦέΗώΑΚΙσΓΘ―ΓœνBΘ§”…Ϋπ τν―‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷ΟΩ…÷ΣΘ§ν― «“Μ÷÷ΫœΜνΤΟΒΡΫπ τΘ§ΗΏΈ¬œ¬Ρή”κΩ’Τχ÷–ΒΡO2Β»Ζ¥”ΠΘ§¥”Εχ≥ωœ÷―œ÷ΊΒΡΑ≤»Ϊ ¬Ι ΓΘ―ΓœνCΘ§ν―ΓΔΟΨΫπ τΨßΧε÷–Θ§‘≠Ή”÷ήΈßΉνΫϋ«“Β»ΨύάκΒΡν―ΓΔΟΨ‘≠Ή” ΐΨυΈΣ12Θ§Υυ“‘Τδ≈δΈΜ ΐΨυΈΣ12ΓΘ―ΓœνDΘ§Ϋπ τΒΡ»έΒψΗΏΒΆ «”…Ϋπ τΦϋΒΡ«Ω»θΨωΕ®ΒΡΓΘ(4)ΗυΨίΓΑΨυΧ·Ζ®Γ±Θ§“ΜΗωΨßΑϊ÷–Κ§”–A‘≠Ή”ΒΡ ΐΡΩΈΣ8ΓΝ1/8ΘΫ1Θ§Κ§”–B‘≠Ή”ΒΡ ΐΡΩΈΣ1Θ§Κ§”–O‘≠Ή”ΒΡ ΐΡΩΈΣ6ΓΝ1/2ΘΫ3Θ§Ι ΗΟΨßΧεΒΡΜ·―ß ΫΈΣABO3ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ6Ϋ≤Μ·―ßΖ¥”ΠΥΌ¬ ΚΆΜ·―ßΤΫΚβΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“ΜΕ®ΧθΦΰœ¬Θ§Ά®Ιΐœ¬Ν–Ζ¥”ΠΩ…“‘÷Τ±ΗΧΊ÷÷Χ’¥…ΒΡ‘≠ΝœMgOΘΚMgSO4(s)ΘΪCO(g) MgO(s)ΘΪCO2(g)ΘΪSO2(g)ΓΓΠΛHΘΨ0ΘΜΗΟΖ¥”Π‘ΎΚψ»ίΒΡΟή±’»ίΤς÷–¥οΒΫΤΫΚβΚσΘ§»τΫωΗΡ±δΆΦ÷–ΚαΉχ±ξxΒΡ÷ΒΘ§÷Ί–¬¥οΒΫΤΫΚβΚσΘ§ΉίΉχ±ξyΥφx±δΜ·«ς ΤΚœάμΒΡ «(ΓΓΓΓ)ΓΘ
MgO(s)ΘΪCO2(g)ΘΪSO2(g)ΓΓΠΛHΘΨ0ΘΜΗΟΖ¥”Π‘ΎΚψ»ίΒΡΟή±’»ίΤς÷–¥οΒΫΤΫΚβΚσΘ§»τΫωΗΡ±δΆΦ÷–ΚαΉχ±ξxΒΡ÷ΒΘ§÷Ί–¬¥οΒΫΤΫΚβΚσΘ§ΉίΉχ±ξyΥφx±δΜ·«ς ΤΚœάμΒΡ «(ΓΓΓΓ)ΓΘ

―Γœνxy
AΈ¬Ε»»ίΤςΡΎΜλΚœΤχΧεΒΡΟήΕ»
BCOΒΡΈο÷ ΒΡΝΩCO2”κCOΒΡΈο÷ ΒΡΝΩ÷°±»
CSO2ΒΡ≈®Ε»ΤΫΚβ≥Θ ΐK
DMgSO4ΒΡ÷ ΝΩ(Κω¬‘ΧεΜΐ)COΒΡΉΣΜ·¬
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ2Ϋ≤Μ·―ß≥Θ”ΟΦΤΝΩΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Ρ≥Ά§―ßΙΚ¬ρΝΥ“ΜΤΩΓΝΓΝ≈ΤΓΑ84œϊΕΨ“ΚΓ±Θ§≤ι‘ΡœύΙΊΉ ΝœΚΆœϊΕΨ“ΚΑϋΉΑΥΒΟςΒΟΒΫ»γœ¬–≈œΔΘΚ
ΓΑ84œϊΕΨ“ΚΓ±ΘΚΚ§25%NaClO 1 000 mLΓΔΟήΕ»1.19 gΓΛcmΘ≠3Θ§œΓ Ά100±Ε(ΧεΜΐ±»)Κσ Ι”ΟΓΘ
«κΗυΨί“‘…œ–≈œΔΚΆœύΙΊ÷Σ ΕΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΗΟΓΑ84œϊΕΨ“ΚΓ±ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ________ molΓΛLΘ≠1ΓΘ
(2)ΗΟΆ§―ß»Γ100 mLΗΟΓΑ84œϊΕΨ“ΚΓ±œΓ ΆΚσ”Ο”ΎœϊΕΨΘ§œΓ ΆΚσΒΡ»ή“Κ÷–c(NaΘΪ)ΘΫ________ molΓΛLΘ≠1(ΦΌ…ηœΓ ΆΚσ»ή“ΚΟήΕ»ΈΣ1.0 gΓΛcmΘ≠3)ΓΘ
(3)Ρ≥ Β―ι–η”Ο480 mLΚ§25%NaClOΒΡœϊΕΨ“ΚΓΘΗΟΆ§―ß≤Έ‘ΡΗΟΓΑ84œϊΕΨ“ΚΓ±ΒΡ≈δΖΫΘ§”ϊ”ΟNaClOΙΧΧε≈δ÷ΤΗΟœϊΕΨ“ΚΓΘ
ΔΌœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________ΓΘ

AΘ°»γ…œΆΦΥυ ΨΒΡ“«Τς÷–Θ§”–ΥΡ÷÷ «≤Μ–η“ΣΒΡΘ§ΜΙ–η“Μ÷÷≤ΘΝß“«Τς
BΘ°»ίΝΩΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσΘ§”ΠΚφΗ…≤≈Ρή”Ο”Ύ»ή“Κ≈δ÷Τ
CΘ°άϊ”ΟΙΚ¬ρΒΡ…ΧΤΖNaClOά¥≈δ÷ΤΩ…ΡήΒΦ÷¬ΫαΙϊΤΪΒΆ
DΘ°–η“Σ≥ΤΝΩΒΡNaClOΙΧΧε÷ ΝΩΈΣ143 g
ΔΎ‘Ύ≈δ÷ΤΙΐ≥Χ÷–Θ§œ¬Ν–≤ΌΉςΩ…Ρή Ι≈δ÷ΤΒΡ»ή“ΚΒΡ≈®Ε»ΤΪ¥σΒΡ «________ΓΘ
AΘ°…’±≠÷–»ή“ΚΉΣ“ΤΒΫ»ίΝΩΤΩ÷– ±Θ§Έ¥œ¥Β”…’±≠
BΘ°Ε®»ί ±Θ§Η© ”ΩΧΕ»œΏ
CΘ°Ε®»ί ±Θ§―ω ”ΩΧΕ»œΏ
DΘ°“Τ“Κ ±Θ§”–…ΌΝΩ“ΚΧεΫΠ≥ω
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ1Ϋ≤Έο÷ ΒΡΉι≥…–‘÷ ΚΆΖ÷άύΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–ΥΒΖ®÷–≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)ΓΘ
AΘ°Ζ÷…Δ÷ ΈΔΝΘ÷±ΨΕΫι”Ύ1ΓΪ100 nm÷°ΦδΒΡΖ÷…ΔœΒ≥ΤΈΣΫΚΧε
BΘ°‘ΎΥ°»ή“ΚάοΜρ»έ»ΎΉ¥Χ§œ¬ΡήΙΜΒΦΒγΒΡΜ·ΚœΈο÷–“ΜΕ® «άκΉ”Μ·ΚœΈο
CΘ°“ΚΧ§¬»Μ·«βΓΔ»έ»Ύ―θΜ·¬ΝΓΔΙΧΧεΝρΥα±ΒΕΦ «ΒγΫβ÷
DΘ°Ζ«Ϋπ τ―θΜ·Έο≤Μ“ΜΕ® «Υα–‘―θΜ·ΈοΘ§”––©Ϋπ τ―θΜ·Έο“≤Ω…Ρή «Υα–‘―θΜ·Έο
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ15Ϋ≤ Β―ιΜ·―ßΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
ΝρΥα―«ΧζΨßΧε(FeSO4ΓΛ7H2O)‘Ύ“Ϋ“©…œΉς≤Ι―ΣΦΝΓΘΈΣ≤βΕ®≤Ι―ΣΦΝ÷–Χζ‘ΣΥΊΒΡΚ§ΝΩΘ§Ρ≥Μ·―ß–Υ»Λ–ΓΉι…ηΦΤΝΥΝΫΧΉ Β―ιΖΫΑΗΘΚ
ΖΫΑΗ“ΜΓΓΒΈΕ®Ζ®ΓΓ”ΟΥα–‘KMnO4»ή“ΚΒΈΕ®≤βΕ®Χζ‘ΣΥΊΒΡΚ§ΝΩΓΘ
Ζ¥”Π‘≠άμΘΚ5Fe2ΘΪΘΪMnO4ΓΣΘΪ8HΘΪ===5Fe3ΘΪΘΪMn2ΘΪΘΪ4H2O
(1) Β―ι«ΑΘ§ Ήœ»“ΣΨΪ»Ζ≈δ÷Τ“ΜΕ®Έο÷ ΒΡΝΩ≈®Ε»ΒΡKMnO4»ή“Κ250 mLΘ§≈δ÷Τ ±–η“ΣΒΡ“«Τς≥ΐΧλΤΫΓΔ≤ΘΝßΑτΓΔ…’±≠ΓΔΫΚΆΖΒΈΙήΆβΘ§ΜΙ–η________(Χν“«ΤςΟϊ≥Τ)ΓΘ
(2)…œ ω Β―ι÷–KMnO4»ή“Κ–η“ΣΥαΜ·Θ§”Ο”ΎΥαΜ·ΒΡΥα «________ΓΘ
AΘ°œΓΝρΥαΓΓΓΓBΘ°≈®œθΥαΓΓΓΓCΘ°œΓœθΥαΓΓΓΓDΘ°œΓ―ΈΥα
(3)Ρ≥Ά§―ß…ηΦΤΒΡœ¬Ν–ΒΈΕ®ΖΫ Ϋ÷–Θ§ΉνΚœάμΒΡ «________(Φ–≥÷≤ΩΖ÷¬‘»Ξ)(ΧνΉ÷ΡΗ–ρΚ≈)ΓΘ

ΖΫΑΗΕΰΓΓ÷ΊΝΩΖ®ΓΓ≤ΌΉςΝς≥Χ»γœ¬ΘΚ

(4)≤Ϋ÷ηΔΎ÷–≥ΐ”ΟH2O2ΆβΜΙΩ…“‘ Ι”ΟΒΡΈο÷ «__________________________ΓΘ
(5)≤Ϋ÷ηΔΎ «ΖώΩ…“‘ Γ¬‘________Θ§άμ”… «________________________________
_______________________________________ΓΘ
(6)≤Ϋ÷ηΔή÷–“ΜœΒΝ–≤ΌΉς“ά¥Έ «ΘΚΙΐ¬ΥΓΔœ¥Β”ΓΔ________ΓΔά以ΓΔ≥ΤΝΩΓΘ
(7)ΦΌ…η Β―ιΈόΥπΚΡΘ§‘ρΟΩΤ§≤Ι―ΣΦΝΚ§Χζ‘ΣΥΊΒΡ÷ ΝΩ________g(”ΟΚ§aΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ14Ϋ≤Έο÷ ΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Ά≠ΓΔΧΦΓΔΒΣΓΔΝρΓΔ¬»Β» «Ήι≥…Έο÷ ΒΡ÷Ί“Σ‘ΣΥΊΓΘ
(1)SΓΔClΉι≥…ΒΡ“Μ÷÷Μ·ΚœΈοΒΡΖ÷Ή”ΫαΙΙ”κH2O2œύΥΤΘ§‘ρ¥ΥΜ·ΚœΈοΒΡΫαΙΙ ΫΈΣ________ΓΘNΓΔOΓΔS»ΐ÷÷‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ____________ΓΘ
(2)Ά≠άκΉ” «»ΥΧεΡΎΕύ÷÷ΟΗΒΡΗ®“ρΉ”Θ§»ΥΙΛΡΘΡβΟΗ «Β±«Α―–ΨΩΒΡ»»ΒψΓΘΡ≥Μ·ΚœΈοY”κCu(Δώ)(Δώ±μ ΨΜ·ΚœΦέΈΣΘΪ1)ΫαΚœ–Έ≥…ΆΦΦΉΥυ ΨΒΡάκΉ”ΓΘ
ΔΌ–¥≥ωCu(Δώ)ΒΡΒγΉ”≈≈≤Φ ΫΘΚ____________ΘΜ
ΔΎΗΟάκΉ”÷–Κ§”–Μ·―ßΦϋΒΡάύ–Ά”–________(Χν–ρΚ≈)ΘΜ
AΘ°ΦΪ–‘Φϋ BΘ°άκΉ”Φϋ CΘ°Ζ«ΦΪ–‘Φϋ DΘ°≈δΈΜΦϋ
ΔέΗΟάκΉ”÷–C‘≠Ή”ΒΡ‘”Μ·ΖΫ Ϋ”–________ΓΘ

(3)œρ¬»Μ·Ά≠»ή“Κ÷–Ά®»κΉψΝΩΒΡΕΰ―θΜ·ΝρΘ§…ζ≥…ΑΉ…Ϊ≥ΝΒμMΘ§MΒΡΫαΙΙ»γΆΦ““Υυ ΨΓΘ–¥≥ωΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_____________________________________
___________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ12Ϋ≤Μ·―ß Β―ιΜυ¥ΓΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
Ρ≥–Υ»Λ–ΓΉιΧΫΨΩSO2ΤχΧεΜΙ‘≠Fe3ΘΪΓΔI2Θ§ΥϊΟ« Ι”ΟΒΡ“©ΤΖΚΆΉΑ÷Ο»γΆΦΥυ ΨΘΚ

(1)SO2ΤχΧεΜΙ‘≠Fe3ΘΪΒΡ≤ζΈο «________(ΧνάκΉ”ΖϊΚ≈)Θ§≤ΈΦ”Ζ¥”ΠΒΡSO2ΚΆFe3ΘΪΒΡΈο÷ ΒΡΝΩ÷°±» «________ΓΘ
(2)œ¬Ν– Β―ιΖΫΑΗ ”Ο”Ύ‘Ύ Β―ι “÷Τ»ΓΥυ–ηSO2ΒΡ «________ΓΘ
AΘ°Na2SO3»ή“Κ”κHNO3 BΘ°Na2SO3ΙΧΧε”κ≈®ΝρΥα
CΘ°ΙΧΧεΝρ‘Ύ¥Ω―θ÷–»Φ…’ DΘ°Ά≠”κ»»≈®H2SO4
(3)ΉΑ÷ΟCΒΡΉς”Ο «____________________________________ΓΘ
(4)»τ“Σ¥”A÷–ΥυΒΟ»ή“ΚΧα»ΓΨßΧεΘ§±Ί–κΫχ––ΒΡ Β―ι≤ΌΉς≤Ϋ÷ηΘΚ’τΖΔΓΔά以ΫαΨßΓΔΙΐ¬ΥΓΔΉ‘»ΜΗ…‘οΘ§‘Ύ’β“ΜœΒΝ–≤ΌΉς÷–ΟΜ”–”ΟΒΫΒΡ“«Τς”–________(Χν–ρΚ≈)ΓΘ
AΘ°’τΖΔΟσΓΓΓΓΓΓBΘ° ·ΟόΆχ
CΘ°¬©ΕΖ DΘ°…’±≠ EΘ°≤ΘΝßΑτ FΘ°έαέω
(5)‘Ύ…œ ωΉΑ÷Ο÷–Ά®»κΙΐΝΩΒΡSO2Θ§ΈΣΝΥ―ι÷ΛA÷–SO2”κFe3ΘΪΖΔ…ζΝΥ―θΜ·ΜΙ‘≠Ζ¥”ΠΘ§ΥϊΟ«»ΓA÷–ΒΡ»ή“ΚΘ§Ζ÷≥…»ΐΖίΘ§≤Δ…ηΦΤΝΥ»γœ¬ Β―ιΘΚ
ΖΫΑΗΔΌΘΚΆυΒΎ“ΜΖί ‘“Κ÷–Φ”»κΥα–‘KMnO4»ή“ΚΘ§ΉœΚλ…ΪΆ »ΞΓΘ
ΖΫΑΗΔΎΘΚΆυΒΎΕΰΖί ‘“Κ÷–Φ”»κKSCN»ή“ΚΘ§≤Μ±δΚλΘ§‘ΌΦ”»κ–¬÷ΤΒΡ¬»Υ°Θ§»ή“Κ±δΚλΓΘ
ΖΫΑΗΔέΘΚΆυΒΎ»ΐΖί ‘“Κ÷–Φ”»κ”ΟœΓ―ΈΥαΥαΜ·ΒΡBaCl2Θ§≤ζ…ζΑΉ…Ϊ≥ΝΒμΓΘ
…œ ωΖΫΑΗ≤ΜΚœάμΒΡ «________Θ§‘≠“ρ «__________________________
______________________________________________ΓΘ
(6)Ρή±μΟςIΘ≠ΜΙ‘≠–‘»θ”ΎSO2ΒΡœ÷œσ «_____________________________
___________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΫ≠Υ’Ή®”Ο ΒΎ11Ϋ≤”–ΜζΜ·―ßΜυ¥ΓΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“ά«ζΧφθΞ «“Μ÷÷ΤΛΖτ≤Ôϓ©Θ§ΥϋΩ…“‘”…‘≠ΝœXΨ≠ΙΐΕύ≤ΫΖ¥”ΠΚœ≥…ΓΘ

œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)ΓΘ
AΘ°‘≠ΝœX”κ÷–ΦδΧεYΜΞΈΣΆ§Ζ÷“λΙΙΧε
BΘ°‘≠ΝœXΩ…“‘ ΙΥα–‘KMnO4»ή“ΚΆ …Ϊ
CΘ°÷–ΦδΧεYΡήΖΔ…ζΦ”≥…ΓΔ»Γ¥ζΓΔœϊ»ΞΖ¥”Π
DΘ°1 mol“ά«ζΧφθΞ÷ΜΡή”κ1 mol NaOHΖΔ…ζΖ¥”Π
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑΧαΖ÷―ΒΝΖ Ή®Χβ9Ζ«Ϋπ τ‘ΣΥΊΦΑΤδΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
»γœ¬ΆΦΥυ ΨΘ§A «“Μ÷÷’ΐ―ΈΘ§B «ΤχΧ§«βΜ·ΈοΘ§C «ΒΞ÷ Θ§F ««ΩΥαΓΘΒ±XΈό¬έ ««ΩΥαΜΙ ««ΩΦν ±ΕΦ”–»γœ¬ΉΣΜ·ΙΊœΒΘ®ΤδΥϊΖ¥”Π≤ζΈοΦΑΖ¥”ΠΥυ–ηΧθΦΰΨυ“―¬‘»ΞΘ©Θ§Β±X ««ΩΦν ±Θ§ΙΐΝΩΒΡBΗζCl2Ζ¥”Π≥ΐ…ζ≥…CΆβΘ§Νμ“Μ≤ζΈο «―ΈΥα―ΈΓΘ

œ¬Ν–ΥΒΖ®÷–≤Μ’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©ΓΘ
AΘ°Β±X ««ΩΥα ±Θ§AΓΔBΓΔCΓΔDΓΔEΓΔF÷–ΨυΚ§Ά§“Μ÷÷‘ΣΥΊΘ§FΩ…Ρή «H2SO4
BΘ°Β±X ««ΩΦν ±Θ§AΓΔBΓΔCΓΔDΓΔEΓΔF÷–ΨυΚ§Ά§“Μ÷÷‘ΣΥΊΘ§F «HNO3
CΘ°BΚΆCl2ΒΡΖ¥”Π «―θΜ·ΜΙ‘≠Ζ¥”Π
DΘ°Β±X ««ΩΥα ±Θ§C‘Ύ≥ΘΈ¬œ¬ «ΤχΧ§ΒΞ÷
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com