
| m |
| V |
| m |
| M |
| a-b |
| 250 |
| n |
| V |
| a-b |
| 250 |
| ag |
| VmL |
| (a-b)g |
| 250g/mol |
| a-b |
| 250 |
| (a-b)g |
| 250g/mol |
| a-b |
| 250 |
| ||
| V��10-3L |
| 4(a-b) |
| V |
| a-b |
| 250 |
| ||
| ag |
| 64(a-b) |
| a |


 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| �ܽ��/g/100gˮ | 18.5 | 20.8 | 26.3 | 32.8 | 40.1 | 48.4 | 52.4 | 50.9 | 43.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| �ܽ��/g/100gˮ | 18.5 | 20.8 | 26.3 | 32.8 | 40.1 | 48.4 | 52.4 | 50.9 | 43.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ij��ѧ��ȤС��������Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�졷ʵ��ʱ���֣�������Һ����������Һ��Ӧʱ����Һ��ɫ������졣Ϊ��̽���˷�Ӧ������С����ԭ��ͬѧ��������ʵ�顣
��1��0.10mol/L H2C2O4��Һ�����ƣ�
ʵ����������80mL 0.10mol/L H2C2O4 ��Һ����Ҫ��ȡ���ᾧ�壨H2C2O4��2H2O g����ȷ��0.1g����ʵ������Ҫ�õ��IJ����������˲���������ͷ�ιܡ���Ͳ��У� ��������ţ�
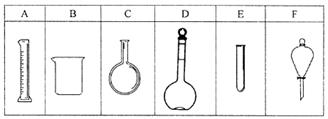
��2����Ӧ���ʱ仯��̽��
| 1���Թ� | 2���Թ� | |
| �����Լ� | 2mL0.10mol/L H2C2O4 ��Һ�� ����ϡ���ᣬ4mL0.010mol/L ��KMnO4��Һ | 2mL0.10mol/L H2C2O4 ��Һ���� ��ϡ���ᣬ4mL0.010mol/L�� KMnO4��Һ������MnSO4���塣 |
| ��ɫʱ�� | 31�� | 4�� |
��H2C2O4��Һ�����Ե�KMnO4��Һ��Ӧ�����ӷ���ʽΪ ��
�ڼ�ͬѧ�ܹ��ó����� ��
��3����һ�������£��ݻ�Ϊ100 L�ܱ������з�����Ӧ��CH4(g)+H20(g)![]() CO(g)+3H2(g)
CO(g)+3H2(g)
 ��H>0����1��O molCH4��2��O mol H 20(g)ͨ����ܱ�������10 minʱ��O��1 mol CO���ɣ���10 min�ڸ÷�Ӧ��ƽ�����ʦ�(H2) ��
��H>0����1��O molCH4��2��O mol H 20(g)ͨ����ܱ�������10 minʱ��O��1 mol CO���ɣ���10 min�ڸ÷�Ӧ��ƽ�����ʦ�(H2) ��
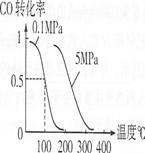
(4)��ѹǿΪO��1 MPa�����£��ݻ�ΪV Lij�ܱ�������amol CO��2amol H2�ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g)![]() CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
�ٸ÷�Ӧ��AH O(�<������>����=��)��
��100��ʱ�÷�Ӧ��ƽ�ⳣ��K (�ú�n��V�Ĵ���ʽ��ʾ)��
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת���� (���������С�����䡱)��ƽ�ⳣ�� (���������С�����䡱)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com