
���� ��1�����ˮ�е��뼸�α���FeCl3��Һ�������������Һ�ʺ��ɫ�����Ƶ�Fe��OH��3���壻��������������Ʊ�ʵ�����Ȼ�����ˮ�⣻�������е������Ƕ����ЧӦ��
��2��Fe��OH��3���������磬���������ƶ���������ɫ�����Ϊ����ĵ�Ӿ��
��3���������Һ��ʹ���巢���۳���
��4�����������ϡ���ᣬ���ȵ��½���ľ۳�������Fe��OH��3�������������������ʹFe��OH��3�����ܽ⣻
��5��������ᴿ��������
��� �⣺��1��ʵ�����Ʊ�������������ԭ�����ڼ��������£�����������ˮ�����������������壬�����Ϊ�����ˮ����μ���FeCl3��Һ��������������Һ�ʺ��ɫ��ֹͣ���ȣ����õ�Fe��OH��3 ���壻��ѧ����ʽΪ��FeCl3+3H2O$\frac{\underline{\;����\;}}{\;}$Fe��OH��3+3HCl���������е������Ƕ����ЧӦ�����ù���������Һ������ж����ЧӦ����˵���������Ƴɣ��ʴ�Ϊ�����ˮ����μ���FeCl3��Һ��������������Һ�ʺ��ɫ��ֹͣ���ȣ����õ�Fe��OH��3 ���壻FeCl3+3H2O$\frac{\underline{\;����\;}}{\;}$Fe��OH��3+3HCl���ù���������Һ������ж����ЧӦ����˵���������Ƴɣ�
��2��Fe��OH��3���������磬����ʯī�缫��ֱͨ���磬ͨ��һ��ʱ���Fe��OH��3�����������ƶ���������ɫ�����Ϊ����ĵ�Ӿ���ʴ�Ϊ�����Fe��OH��3���������磻��Ӿ��
��3�������м��뱥�ͣ�NH4��2SO4��Һ�������Fe��OH��3������ԭ���ǵ������Һ��ʹ���巢���۳����ʴ�Ϊ�����ɳ������������Һ��ʹ���巢���۳���
��4������ϡ�����ǵ������Һ���ʿ�ʼ����ϡ���ᣬ�ᵼ�½���ľ۳�������Fe��OH��3�������������������ʹFe��OH��3�����ܽ⣬�ʴ�Ϊ�������ɳ������������ʧ����������ϡ���ᷢ������ľ۳�����ϡ���Ὣ���ɵ��������������ܽ⣻
��5��������ᴿ���������ʴ�Ϊ��������
���� ���⿼����Fe��OH��3 ������Ʊ��ͽ�������ʣ���Ŀ�ѶȲ����״���Ϊ������Ʊ�ԭ����ע���Ʊ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ��Ŀ�� |
| A | ����KSCN��Һ��FeSO4��Һ�еμ������ữ��H2O2��Һ | ����H2O2�������Դ���Fe3+ |
| B | ������ͨ��ϡ��ˮ�� | ����SO2 ���Ƿ������ϩ |
| C | ������ɫʯ����Һ | ����ƾ����Ƿ���д��� |
| D | �ñ���̼������Һϴ�Ӻ��Һ | ��ȥ�����������������Ҵ������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������ȱ�����������֯��WHO��ȷ��Ϊ���Ĵ�A1����������
�������ȱ�����������֯��WHO��ȷ��Ϊ���Ĵ�A1�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����0.1mol•L-1 NaOH��Һʱ���ó�ʪ���ձ�����NaOH���壬�����ƽ����Ӱ�� | |
| B�� | ����һ�����ʵ���Ũ����Һʱ������Ͳ��ȡŨ��Һ������Ӷ�����������Һ��Ũ�Ƚ��ƫ�� | |
| C�� | 98%��Ũ�����õ������ˮϡ�ͺ��������������Ϊ49% | |
| D�� | �¶�һ��ʱ����ʳ��ˮ�м�������Ȼ��ƣ�����Һ��Ũ�Ⱥ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | W10O21 | B�� | W8O22 | C�� | W10O27 | D�� | W5O14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
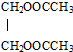 ���������������Ѻ��ԣ�
���������������Ѻ��ԣ� ��
��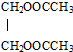 �ķ�Ӧ����ʽCH3COOH+HOCH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$
�ķ�Ӧ����ʽCH3COOH+HOCH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$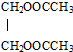 +2H2O��
+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com