
| ���������� | �¶����䣨K�� | ������g�� |
| Sc2��C2O4��3•6H2O | 298 | 0.462 |
| 383��423 | 0.372 | |
| 463��508 | 0.354 | |
| 583��873 | 0.138 |
���� ��1�������ֵ�ԭ����������̬ԭ�Ӻ�������Ų�ʽ�ж���δ�ɶԵ����������ж�����ͬ�����Һ�����ͬδ�ɶԵ������ķǽ���Ԫ�أ�
��2�����Ӿ����۷е�ߵ�ȡ�������Ӽ���ǿ�������Ӽ�ǿ�����������Ӱ뾶�ʹ�С�йأ�
��3���Ȼ��ƺ��Ȼ��ؾ��嶼�������Ӿ��壬���ߵ���������ͬ���������Ӱ뾶С�ڼ����ӣ����Ȼ��Ƶľ����ܴ����Ȼ��أ�
��4��ˮ���ӵ�����ԭ��O�γ�������O-H����������2�Թ¶Ե��ӣ����ӻ���ʽΪsp3�ӻ���
��5�����ݱ������ݼ�������¶ȶη��������仯��ԭ�Ӷ��ó�Sc2��C2O4��3•6H2O�е�ˮ�������ࣻ
��6��Sc2��C2O4��3•6H2O��583K���ȵ�873K�����ѵĻ�ѧ���ɲ����������������֮������Ӽ�������������еĹ��ۼ���
��� �⣺��1����Ϊ21��Ԫ�أ���̬ԭ�Ӻ�������Ų�ʽΪ��1s2 2s2 2p6 3s2 3p6 3d1 4s2��λ�ڵ������ڢ�B�壬��δ�ɶԵ�����Ϊ1���ڵ��������У�δ�ɶԵ�����Ϊ1�ķǽ���Ԫ��ΪBr��
�ʴ�Ϊ��Br��
��2������ͼ1��֪���ֵĵ����ܢ�1����2����3�仯��С��������4ʱ�仯�ϴ�����$\frac{{I}_{2}}{{I}_{1}}$�ı�ֵС��$\frac{{I}_{4}}{{I}_{3}}$��
�ʴ�Ϊ������
��3��������������Ӵ������ͬ�������Ӱ뾶С������Ӱ뾶�������������Ӽ�ǿ�������۵�Ҫ���ߣ�
�ʴ�Ϊ�������Ӱ뾶С�ڼ����ӣ��Ȼ��ƾ����ܴ����Ȼ��أ������Ȼ����۵�ߣ�
��4��H2O�����е�����ԭ��Oԭ���γ���2��������������������µ��Ӷԣ�����Oԭ�Ӳ���sp3�ӻ�������H2O������Oԭ���ṩsp3�ӻ�����γ�H-O �Ҽ�
�ʴ�Ϊ��sp3�ӻ���
��5��0.462g Sc2��C2O4��3•6H2O�����ʵ���Ϊ��$\frac{0.462g}{462g/mol}$=0.001mol��383��423Kʱʧȥˮ�����ʵ���Ϊ��$\frac{0.462g-0.372g}{18g/mol}$=0.005mol��ʧȥˮ����ĿΪ��$\frac{0.005mol}{0.001}$=5��463��508Kʱʧȥˮ�����ʵ���Ϊ��$\frac{0.372g-0.354g}{18g/mol}$=0.001mol��ʧȥˮ����ĿΪ��$\frac{0.001mol}{0.001mol}$=1����ʱ Sc2��C2O4��3•6H2O�е�ˮ��ȫʧȥ��������ɵ�����Ϊ0.1molSc2O3������Ϊ��138g/mol��0.001mol=0.138g�������������Ǻϣ�����Sc2��C2O4��3•6H2O�е�ˮ���ӿ��Է�Ϊ2�֣�
�ʴ�Ϊ��2��
��6�����ݣ�5���ļ����֪��Sc2��C2O4��3•6H2O��583K���ȵ�873K�����ѵĻ�ѧ���У������������������֮������Ӽ�������������еĹ��ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
���� ���⿼����λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰Ԫ�������������ڱ���Ӧ�á���ѧ�����ͼ��ӻ���ʽ��֪ʶ��ע����������ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�������ɵĹ�ϵ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ�����Ӧ�û���֪ʶ��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuO | B�� | H2SO4 | C�� | CuSO4 | D�� | Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
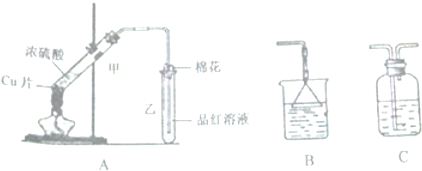
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
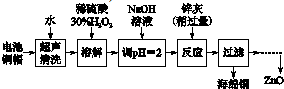
| ��ʼ������pH | ������ȫ��pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���͡����͡�ţ�͡�ֲ���͵������������� | |
| B�� | �����̼ԭ�ӵ��л�������������γ��ĸ�̼̼���� | |
| C�� |  ��ij�л�����H2�����ӳɷ�Ӧ��IJ�����ϸ��������ȶ��л��ﹲ��3�� ��ij�л�����H2�����ӳɷ�Ӧ��IJ�����ϸ��������ȶ��л��ﹲ��3�� | |
| D�� | �ṹƬ��Ϊ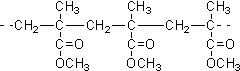 �ĸ߾�����䵥��ͨ�����۷�Ӧ���� �ĸ߾�����䵥��ͨ�����۷�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
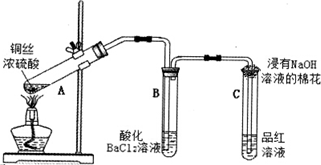
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ð�ˮ�������� | |
| B�� | ��ˮϡ�����У�c��H+��/c��OH-����ֵ��С | |
| C�� | ��ͬ����pH=11��NaOH��Һ��ȣ�NaOH��Һ��c��Na+�����ڰ�ˮ��c��NH4+�� | |
| D�� | ��������NH4Cl ���壬��Һ��ˮ�ĵ���ƽ�⣺H2O?H++OH-�����ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ�ȣ�mol/L�� | 3��10-6 | 7��10-6 | 2��10-5 | 3��10-5 | 5��10-5 | 2��10-5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com