
þ�ǡ��������������Ӻ�ˮ����ȡþͨ��Ҫ�������������ͼ:

��ش���������:
(1)����1����ߺ�ˮ��Mg2+Ũ�ȣ������� ��
(2)����2�����ӷ���ʽΪ ��
��֪Mg(OH)2��Ksp��5.61��10-12������Ũ����ˮ��MgCI2Ũ��Ϊ3mo1/L����ҪʹMg2+�γ�Mg( OH )2������������������ˮ�е�OH-Ũ������Ҫ�ﵽ ��
��ҵ����Ϊ�˻�ø��ߵ������ݱ�1������������ij�������_ ���������ֳ������ķ����� ��
��1�Լ��۸�
|
�Լ� |
KOH |
NaOH |
Ca(OH)2 |
|
�۸�Ԫ���֣� |
6800 |
3200 |
1200 |
(3)����3���ʵ��?��λͬѧ������з���:
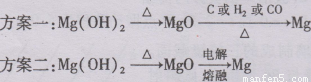
�����ַ����Ƿ����?��������:
����һ: ��
������: ��
�����Ʒ�����ʲô?д������ͼ:
��
��15�֣���1��ȡ��ˮɹ�κ�Ŀ�±ˮ��ԭ�ϣ����������𰸸��֣�2�֣�
��2��Mg2����2OH����Mg(OH)2����Mg2����Ca(OH)2��Mg(OH)2����Ca2����2�֣���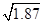 ��10��6mol/L��2�֣��ش�1.36��10��6mol/LҲ�ɣ���Ca(OH)2��1�֣����Ժ��ߵı��Ǹ��·ֽ�����ʯ�ң���ʯ����ˮ��Ӧ�Ƶ�ʯ���飨2�֣�
��10��6mol/L��2�֣��ش�1.36��10��6mol/LҲ�ɣ���Ca(OH)2��1�֣����Ժ��ߵı��Ǹ��·ֽ�����ʯ�ң���ʯ����ˮ��Ӧ�Ƶ�ʯ���飨2�֣�
��3������һ����������H2��C��CO�����ܻ�ԭMgO��2�֣�
����������������MgO�۵�ߣ��ܺĴ����ã�2�֣�
���������� ��2�֣�
��2�֣�
��������
�����������1����ˮMg2+��Ũ�Ⱥ�С�������ߺ�ˮ��Mg2+Ũ�ȵķ�����ȡ��ˮɹ�κ�Ŀ�±ˮ��ԭ�ϡ�
��2������2��Ŀ���ǽ�Mg2+ת��Ϊ������þ�����������йط�Ӧ�����ӷ���ʽΪMg2����2OH����Mg(OH)2����Mg2����Ca(OH)2��Mg(OH)2����Ca2��������������þ���ܶȻ�������֪��Ũ����ˮ��MgCI2Ũ��Ϊ3mo1/L��ҪʹMg2+�γ�Mg( OH )2������������������ˮ�е�OH-Ũ������Ҫ�ﵽ ��
��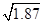 ��10��6mol/L���Ӿ���Ч�����Դ������������ij�����Ӧ�����������ơ������������Ƶķ������Ժ��ߵı��Ǹ��·ֽ�����ʯ�ң���ʯ����ˮ��Ӧ�Ƶ�ʯ���顣
��10��6mol/L���Ӿ���Ч�����Դ������������ij�����Ӧ�����������ơ������������Ƶķ������Ժ��ߵı��Ǹ��·ֽ�����ʯ�ң���ʯ����ˮ��Ӧ�Ƶ�ʯ���顣
��3������H2��C��CO�����ܻ�ԭMgO���Է���һ������������ΪMgO�۵�ߣ��ܺĴ����ã���˷�����Ҳ����������ȷ�ķ���Ӧ���ǽ�������þת��Ϊ�Ȼ�þ��Ȼ�������ڵ��Ȼ�þ���ɡ�����ͼ���£�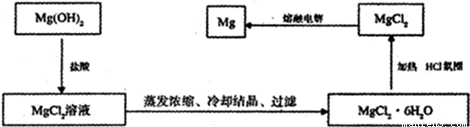 ��
��
���㣺���麣ˮ��þ�ᴿ���й�ʵ����ơ��������ж��Լ����ۣ��ܶȻ��������йؼ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ����þ | �Ȼ�þ |
| �۵�/�� | 2852 | 714 |
A����ˮ
| ||||||
B����ˮ
| ||||||
C����ˮ
| ||||||
D����ˮ
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�ҹ����ŷḻ�ĺ�ˮ��Դ����ˮ����Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����CO32����HCO3�������ӡ�þ����Ͻ�����Խ�����������ɻ����췽�����Ҫ���С�����������֮�ơ�������Ҷ����Ӻ�������þ���й���ˮ��þ����Ҳ�ڲ��Ͻ�������ˮ���京þ���Ӻܶ࣬�����Ǻܷ�ɢ�ģ�1.28 g?L-1����Ҫ��ȡþ����Ӧ��þ�������������ӵȷ��롣
��ش��������⣺
��1��ij��ѧ��ȤС���������պ��߱������ò��Ͷ��Ũ���ĺ�ˮ�У����ձ����Mg(OH)2��Һ����д�����һ����Ӧ�����ӷ���ʽ�� ��
��2����֪������þ�۵�2852�棬�Ȼ�þ���۵�714�档��ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д��ʵ���������Ȼ�þ��Һ��ȡ��ˮ�Ȼ�þ�����ʵ�������� ��ijͬѧ�������õ��Ĺ��壬�þƾ���Ƽ���ȴ�����ۻ��������Է���ԭ��

��3�����������ڽ��յ���Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ�� ��
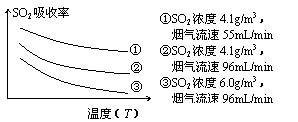 ��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ��������ͼ��ʾ���������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺 ��
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ��������ͼ��ʾ���������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺 ��
����Ȼ��ˮ�����˺��������������H2SO3��HSO3���ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ���� ����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com