�����仯�����ڹ��õķ�չ��������Ҫ���ã�
��1����֪��4Fe��s��+3O
2��g��=2Fe
2O
3��s����H=-1641.0kJ?mol
-1C��ʯī��+1/2O
2��g��=CO��g����H=-110.5kJ?mol
-1��Fe
2O
3��s��+3C��ʯī��=2Fe��s��+3CO��g���ġ�H=
+489.0kJ/mol
+489.0kJ/mol
kJ?mol
-1��
��2�����ڳ�ʪ�Ŀ����������绯ѧ��ʴ��ijͬѧ��NaCl��Һ����һ�����������������ϣ�һ��ʱ�����Һ�θ��ǵ�Բ����������a���ѱ���ʴ���䰵����Һ�������γ���ɫ�����b������ͼ1��ʾ��Һ�α�Ե��
����
����
�����������������������缫��ӦʽΪ
O2+2H2O+4e-=4OH-
O2+2H2O+4e-=4OH-
��
��3�����ѺϽ���һ�ֳ��õIJ���ֲ��ϣ�ijͬѧ��̽���úϽ������ʱ��������TiO
2+��Fe
3+��Һ�м�����м����Һ����ɫ���ù����з����ķ�Ӧ�У�
��2TiO
2+����ɫ��+Fe+4H
+=2Ti
3+����ɫ��+Fe
2++2H
2O
��Ti
3+����ɫ��+Fe
3++H
2O=TiO
2+����ɫ��+Fe
2++2H
+��
2Fe3++Fe=3Fe2+
2Fe3++Fe=3Fe2+
��
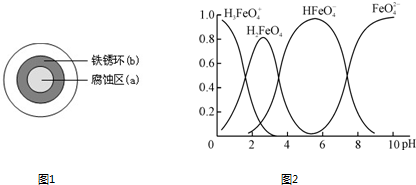
��4���ٸ�����أ�K
2FeO
4����һ��������ˮ��������
��ˮ��Һ�еĴ�����̬��ͼ2��ʾ���������ʾ��������̬�ķ����ֲ���
����˵������ȷ����
AB
AB
��������ĸ��
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B����pH=10��������Һ�м�������pH=2��HFe
�ķֲ�����������
C����pH=6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFe
+OH
-�TFe
+H
2O
��K
2FeO
4����ˮ��ų�һ����ɫ��ζ���壬��ɱ������������ˮ�е��������ʵ�ԭ���������ӷ���ʽ��ʾΪ
4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��
4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��
��
��5����һ������Fe��FeO��Fe
3O
4�Ļ�����м���100mL 1mol?L
-1�����ᣬǡ��ʹ�������ȫ�ܽ⣬�ų�224mL����״�������壬����KSCN��Һ���Ժ�ɫ������������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ɵ���
2.8
2.8
g��


 �������ȼҵ����Ҫ��Ʒ֮һ����һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
�������ȼҵ����Ҫ��Ʒ֮һ����һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
