±±¾©Ź±¼ä2008Äź9ŌĀ25ČÕ21Ź±10·Ö04Ćė£¬ĪŅ¹ś×ŌŠŠŃŠÖʵÄÉńÖŻĘßŗÅŌŲČĖ·É“¬ŌŚ¾ĘČŖĪĄŠĒ·¢ÉäÖŠŠÄ·¢ÉäÉżæÕ£®µ£ČĪÉńĘß·É“¬·¢ÉäČĪĪńµÄ³¤Õ÷¶žŗÅFŌĖŌŲ»š¼ż£®»š¼żĶĘ½ų¼ĮŹĒĘ«¶ž¼×ėĀ{·Ö×ÓŹ½£ŗC
2H
8N
2}ÓėĖÄŃõ»Æ¶žµŖ£®
£Ø1£©6.0gŅŗĢ¬Ę«¶ž¼×ėĀÓė×ćĮæµÄŅŗĢ¬ĖÄŃõ»Æ¶ž»ÆµŖĶźČ«·“Ӧɜ³ÉN
2£Øg£©”¢CO
2£Øg£©”¢H
2O£Øg£©”¢·Å³ö225.0kJµÄČČĮ森
¢ŁŠ“³öÉĻŹö·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ
£®
¢ŚĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ
A”¢ĪļÖŹµÄČ¼ÉÕ·“Ó¦±ŲŠėÓŠŃõĘų²Ī¼Ó
B”¢øĆ·“Ó¦ÖŠŃõ»Æ¼ĮÓė»¹Ō¼ĮĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1
C”¢Ę«¶ž¼×ėĀČ¼ÉÕČČĪŖ2250kJ/mol
D”¢Ę«¶ž¼×ėĀÓėĖÄŃõ»Æ¶žµŖ×÷»š¼żĶĘ½ų¼ĮŹĒ¶žÕß·¢Éś¾ēĮŅµÄŃõ»Æ»¹Ō·“·Å³ö“óĮæµÄČČŗĶ²śÉś“óĮæĘųĢåµÄŌŅņ
£Ø2£©Ź¹ÓĆĘ«¶ž¼×ėĀÓėĖÄŃõ»Æ¶žµŖ×÷Č¼ĮĻ£¬Ź¹·¢Éä³”²śÉś×Ų»ĘÉ«”°Ä¢¹½”±ŌĘĆÖĀž£¬Ėšŗ¦¹¤×÷ČĖŌ±µÄÉķĢ彔浣¬“ųĄ“Ņ»Š©ŗóŅÅÖ¢£®²śÉś×Ų»ĘÉ«”°Ä¢¹½”±ŌʵÄæÉÄÜŌŅņ
£®
£Ø3£©ŠĀµÄ»·±£µĶĪĀČ¼ĮĻ¼Ę»®ŌŚ2014Äźµ½2016ÄźŹ¹ÓĆ£¬½«ŌŚŗ£ÄĻ·¢Éä³”·¢ÉäÖŠµČĶĘĮ¦»š¼żµÄŹ±ŗņ²ÉÓĆ£¬ŠĀµÄµĶĪĀČ¼ĮĻŹĒŅŗĒā£®ĄūÓĆŗĖÄÜ°ŃĖ®·Ö½ā£¬ÖĘČ”ĒāĘų£¬ŹĒÄæĒ°Šķ¶ą¹ś¼ŅÕżŌŚŃŠ¾æµÄæĪĢā£®ĻĀĶ¼ŹĒ¹śĶāÕżŌŚŃŠ¾æµÄŅ»ÖÖĮ÷³Ģ£ØĮņ-µāČČŃ»··Ø£©£¬ĘäÖŠÓĆĮĖ¹żĮæµÄµā£®
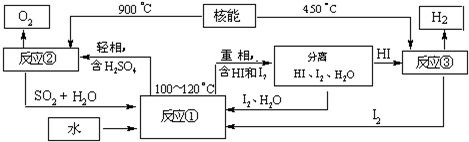
Š“³ö¢Ł·“Ó¦µÄ»Æѧ·½³ĢŹ½
£»
ÓĆĮņ-µāČČŃ»··ØÖĘČ”Ēā×ī“óµÄÓŵćŹĒ
£®



 Š”Ģāæń×öĻµĮŠ“š°ø
Š”Ģāæń×öĻµĮŠ“š°ø
