
 ���仯������о�������ѧ�ķ�չ��ռ�ж��صĵ�λ��
���仯������о�������ѧ�ķ�չ��ռ�ж��صĵ�λ�� H++B��OH��4-
H++B��OH��4-���� ��1��Gaԭ�Ӻ��������Ϊ31������������ԭ����д��̬ԭ�Ӻ�������Ų�ʽ��
ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�NԪ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ�
��2��������[B��OH��3]�����У�Bԭ����3���ǻ�������Bԭ��û�й¶Ե��ӣ��ӻ������ĿΪ3��
���ᾧ�������ʯī���ƵIJ�״�ṹ����ͬ��֮��Ϊ���»�����ͬ����Ӽ�Ϊ�����
��3��H3BO3������KOH��Һ��Ӧ�����ӷ���ʽΪH3BO3+OH-�TB��OH��4-������ΪһԪ���ᣬ��������B��OH��4-�������ӣ�
��4�����⻯�ƣ�NaBH4������ˮ��ˮ�����������ƺ�������[BH4]-��Bԭ�ӹµ��Ӷ���=$\frac{3+1-1��4}{2}$=0���۲���Ӷ���=4+0=4��
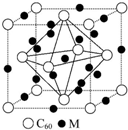
��5�����ݾ�̯�����㾧��֮��Mԭ����Ŀ��C60������Ŀ������ȷ����ѧʽ��
��� �⣺��1��Gaԭ�Ӻ��������Ϊ31�����������ԭ������֪��̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d104s24p1��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�NԪ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ������Ϊ��N��O��B��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��N��O��B��
��2��������[B��OH��3]�����У�Bԭ����3���ǻ�������Bԭ��û�й¶Ե��ӣ��ӻ������ĿΪ3��������Bԭ���ӻ������������sp2�����ᾧ�������ʯī���ƵIJ�״�ṹ����ͬ��֮��Ϊ���»�����ͬ����Ӽ���ԭ������ԭ��֮���γ������
�ʴ�Ϊ��sp2�������
��3��H3BO3������KOH��Һ��Ӧ�����ӷ���ʽΪH3BO3+OH-�TB��OH��4-������ΪһԪ���ᣬ��������B��OH��4-�������ӣ����뷽��ʽΪ��H3BO3+H2O  H++B��OH��4-��
H++B��OH��4-��
�ʴ�Ϊ��H3BO3+H2O  H++B��OH��4-��
H++B��OH��4-��
��4�����⻯�ƣ�NaBH4������ˮ��ˮ�����������ƺ���������Ӧ����ʽΪ��NaBH4+4H2O�TNa[B��OH��4]+4H2����[BH4]-��Bԭ�ӹµ��Ӷ���=$\frac{3+1-1��4}{2}$=0���۲���Ӷ���=4+0=4���ռ�ṹΪ���������Σ���
�ʴ�Ϊ��NaBH4+4H2O�TNa[B��OH��4]+4H2�����������壻
��5��������Mԭ��λ�ھ������������ڲ���������12��M���ڲ���9��M���þ�����Mԭ�ӵĸ���Ϊ 12��$\frac{1}{4}$+9=12��C60���ڶ��������ģ�����C60��ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Mԭ�Ӻ�C60���ӵĸ�����Ϊ3��1����ò��ϵĻ�ѧʽΪM3C60��
�ʴ�Ϊ��12��M3C60��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ��ӻ���ʽ��ռ�ṹ�жϡ������йؼ����Լ���Ϣ��ȡ��Ǩ�����õȣ���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO3 | B�� | N2O5 | C�� | Na2O | D�� | CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
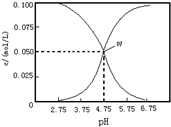
| A�� | ���¶��´���ĵ���ƽ�ⳣ��Ϊka=10-4.75 | |
| B�� | pH=6����Һ�У�c��K+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol/L | |
| C�� | pH=3.75����Һ��c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� | |
| D�� | ��W����ʾ��Һ��ͨ��0.1molHCl���壨��Һ������Ժ��Բ��ƣ�c��H+��=c��OH-��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�������� | |
| B�� | Fe3+�������Դ���Cu2+ | |
| C�� | ��Һ��Cu2+��Fe2+�����ʵ�����Ϊ1��1 | |
| D�� | 1molFe�ɻ�ԭ2molFe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ư��Һ��ͨ������CO2��2ClO-+CO2+H2O�T2HClO+CO32- | |
| B�� | �ð�ˮ����������������SO2+NH3•H2O�THSO3-+NH4+ | |
| C�� | ������������������Һ���ն���������3NO2+2OH-�T2NO3-+NO+H2O | |
| D�� | ���������Һ��ͨ������SO2���壺Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʷе㣺W��Y | |
| B�� | X������������Ӧ��ˮ��������Y�������ﷴӦ | |
| C�� | W��X�γɵĻ������к��й��ۼ� | |
| D�� | �����ӵİ뾶��X��Y��Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ζ��ܡ���Һ���Լ��ζ�����������ʢ����Һ����ƿ��ʹ��ǰ����Ҫϴ������ϴ | |
| B�� | ʵ������ȡ����ʱ����װ�ö������̣��ټ��װ�õ������� | |
| C�� | �����ɫש�����Ƿ��������IJ���Ϊ����Ʒ���������ˮ�ܽ�����ˡ�����Һ�еμ�KSCN��Һ | |
| D�� | ���к��Ȳⶨ��ʵ���У�������������Һ�������Ϸ�Ӧ�������¶���Ϊĩ�¶� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com