
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
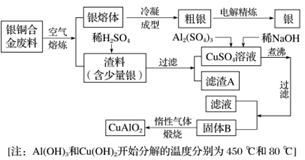
(1)��⾫����ʱ��������ӦʽΪ___________________________________________________��
����A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ��Ӧ����ʽΪ____________��
(2)��������B�����Ϊ__________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ____________��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ��____CuO��____Al2O3 ____CuAlO2��________����
____CuAlO2��________����
(4)����ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0 kg�����е�ͭ����ȫת��Ϊ________ mol CuAlO2��������Ҫ 1.0 mol·L��1��Al2(SO4)3��Һ________ L��
(5)CuSO4��ҺҲ�������Ʊ������������������________�����ˡ�ϴ�Ӻ��
�𰸡�(1)Ag����e��===Ag��2NO��O2===2NO2
(2)Al(OH)3��Cu(OH)2��Al(OH)3��OH��===AlO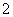 ��2H2O
��2H2O
(3)4��2��4��O2
(4)50��25
(5)�����ᾧ
���������ݵ��ԭ�������ʵ����ʼ��������̡�������ԭ��Ӧ���غ����ȷ�����
(1)��⾫����ʱ��������ӦʽΪAg����e��===Ag�������ɫ�����ķ�ӦΪ2NO��O2===2NO2��(2)����B����Cu(OH)2��Al(OH)3����NaOH��������Al(OH)3��ת��ΪNaAlO2��(3)�÷�ӦΪ������ԭ��Ӧ�����ݵ�ʧ�����غ㡢ԭ���غ�ȷ��ȱ�����ʣ�Ȼ����ƽ��ѧ����ʽ��(4)����CuAlO2�����ʵ���Ϊ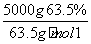 ��50 mol����AlԪ���غ��֪��������ҪAl2(SO4)3�����ʵ���Ϊ 25 mol��������Һ���Ϊ25 L��(5)CuSO4�ӱ�����Һ�нᾧ����CuSO4·5H2O(����)����CuSO4��Һ�Ʊ�������Ҫ�IJ���Ϊ�����ᾧ�����ˡ�ϴ�Ӻ��
��50 mol����AlԪ���غ��֪��������ҪAl2(SO4)3�����ʵ���Ϊ 25 mol��������Һ���Ϊ25 L��(5)CuSO4�ӱ�����Һ�нᾧ����CuSO4·5H2O(����)����CuSO4��Һ�Ʊ�������Ҫ�IJ���Ϊ�����ᾧ�����ˡ�ϴ�Ӻ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ����������ȷ����(����)
A���ǽ���Ԫ����ɵĻ�������ֻ�����ۼ�
B��C��N��O��H����Ԫ���γɵĻ�����һ���������Ӽ������й��ۼ�
C����ͬԪ�ص�ԭ�ӹ��ɵķ���ֻ�����Թ��ۼ�
D��CH4�����еļ۵��Ӷ������γɹ��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.102 6 mol·L��1������ζ�25.00 mLδ֪Ũ�ȵ�����������Һ���ζ����յ�ʱ���ζ����е�Һ����ͼ��ʾ����ȷ�Ķ���Ϊ(����)

A��22.30 mL B��22.35 mL
C��23.65 mL D��23.70 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͻ��봿�����ƳɵĽ���������ȣ��ŵ���(����)
�ٺϽ��Ӳ��һ������ĸ��ɷֽ����Ĵ�
��һ��أ��Ͻ���۵�����ĸ��ɷֽ����ĸ���
�۸ı�ԭ�ϵ���ȡ��ı����ɺϽ���������õ��в�ͬ���ܵĺϽ�
�ܺϽ�ȴ������ĵ����Ը�ǿ
�ݺϽ�ȴ�������Ӧ�÷�Χ���㷺
A���ڢۢ� B���٢ڢۢ�
C���٢ڢ� D���٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ĩ״����A���ɵ����ʵ�����MgO��Fe2O3��ɵĻ�����������ʵ�飺
��ȡ����A�������ȷ�Ӧ���������е���B���ɣ�
����ȡ20 g Aȫ������0.15 L 6.0 mol·L��1�����У�����ҺC��
�۽����еõ��ĵ���B����ҺC��Ӧ���ų�1.12 L(���)���壬ͬʱ������ҺD���������й�������B��
����KSCN��Һ����ʱ����ҺD����ɫ��
����գ�
(1)�����������ȷ�Ӧ��ʵ�������________�������еĵ���B��________��
(2)�����������ĸ���Ӧ�Ļ�ѧ����ʽ��__________________________________________
________________________________________________________________________��
(3)�����������ĸ���Ӧ�����ӷ���ʽ��____________________________________
________________________________________________________________________��
(4)����ҺD���������Ϊ0.15 L�������Һ��c(Mg2��)Ϊ__________��c(Fe2��)Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ν�Ͻ𣬾��Dz�ͬ�ֽ���(Ҳ����һЩ�ǽ���)���ۻ�״̬���γɵ�һ���ۺ���±�Ϊ���ֽ������ۡ��е㣺
| Na | Cu | Al | Fe | |
| �۵�(��) | 97.5 | 1 083 | 660 | 1 535 |
| �е�(��) | 883 | 2 595 | 2 200 | 3 000 |
�������������ж����в����γɺϽ����(����)
A��Cu��Al B��Fe��Cu
C��Fe��Na D��Al��Na
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70% Cu��25% Al��4% Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
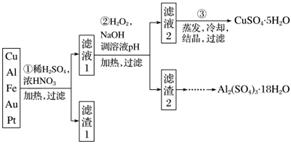
��ش��������⣺
(1)�ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ____________________________________________
________________________________________________________________________��
�õ�����1����Ҫ�ɷ�Ϊ____________��
(2)�ڢڲ���H2O2��������______________��ʹ��H2O2���ŵ���______________������ҺpH��Ŀ����ʹ______________���ɳ�����
(3)�õڢ۲�����CuSO4·5H2O�Ʊ���ˮCuSO4�ķ�����___________________________��
(4)������2��ȡAl2(SO4)3·18H2O ��̽��С����������ַ�����
�ף�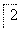
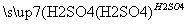
 ,��������ȴ���ᾧ������
,��������ȴ���ᾧ������
Al2(SO4)3·18H2O
�ң�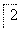
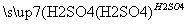

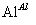

,��������ȴ���ᾧ������Al2(SO4)3·18H2O
����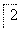
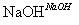

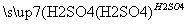

,��������ȴ���ᾧ������Al2(SO4)3·18H2O
�������ַ����У�________���������У�ԭ����________________________________
________________________________________________________________________��
��ԭ�������ʽǶȿ��ǣ�__________������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�߿�ѡ�������жϣ���ȷ�Ĵ̡�������Ĵ���
(1)SiO2��NaOH��HF�����������ܷ�Ӧ(����)
(2014·���գ�8C�ı�)
(2)Si��SiO2������������ά(����)
(2014·���ϣ�10D)
(3)�轺������װʳƷ�ĸ����(����)
(2014·�Ĵ����ۣ�1B)
(4)SiO2������KOH��Һ��Ӧ������Ũ���ᷴӦ(����)
(2014·�������ۣ�9�ڸı�)
(5)��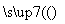 ��
��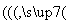 ��������Ϊ��̿��������O2(����)
��������Ϊ��̿��������O2(����)
(2013·���գ�6A)
(6)�ϳ���ά���ά�����������ǽ�������(����)
(2012·�¿α�ȫ������8D)
(7)SiO2����HF��Ӧ����������ܱ����ڲ���ƿ��(����)
(2013·�㶫���ۣ�10D)
(8)�������ý�̿��ԭSiO2��ȡ�ֹ�(����)
(2013·�㶫���ۣ�11C)
(9)��������ҺӦ�����ڴ����������Լ�ƿ��(����)
(2012·���ϣ�4B)
(10)ˮ���������������ϼ��ͷ����(����)
(2010·���գ�4B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ����A��ͨ���������ر�B��ʱ��C������ĺ첼������������������B����C������ĺ첼������ɫ����Dƿ��ʢ�ŵ���Һ��������(����)
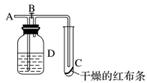
A��Ũ����
B��NaOH��Һ
C������Na2SO3��Һ
D�������Ȼ�����Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com