���㣺���ӷ���ʽ���йؼ���,�йػ���ﷴӦ�ļ���
ר�⣺
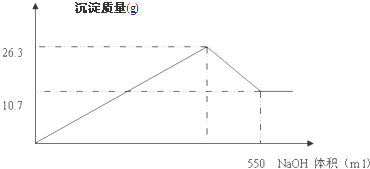
��������1���Ȼ������������Ʒ�Ӧ�������������������Ȼ��ƣ��Ȼ��������������������Һ��Ӧ����ƫ��������ˮ��
��2�����ճ���10.7gΪFe��OH��
3�����ȷֽ�����Fe
2O
3������n=
���������������ʵ���������FeԪ���غ����n��Fe
2O
3�����ٸ���m=nM����m��Fe
2O
3����
��3������FeԪ���غ����n��FeCl
3��=n[Fe��OH��
3]���ٸ���c=
����c��FeCl
3����
��4���������ʱΪ26.3g��Ϊ������������������������֮�ͣ�������������������������n=
�����������������ʵ���������AlԪ���غ����n��AlCl
3�����ٸ���c=
����c��AlCl
3����
��5������550mL NaOH��Һ��ʱ����Һ������ΪNaCl��NaAlO
2�������������غ�n��NaCl��=3n��FeCl
3��+3n��AlCl
3��������AlԪ���غ�n��NaAlO
2��=n��AlCl
3���������������غ�n��NaOH��=n��NaCl��+n��NaAlO
2�����ٸ���c=
����c��NaOH����
���
�⣺��1���Ȼ������������Ʒ�Ӧ�������������������Ȼ��ƣ���Ӧ���ӷ���ʽΪ��Fe
3++3OH
-=Fe��OH��
3�����Ȼ��������������������Һ��Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al
3++4OH
-=AlO
2-+2H
2O��
�ʴ�Ϊ��Fe
3++3OH
-=Fe��OH��
3����Al
3++4OH
-=AlO
2-+2H
2O��
��2�����ճ���10.7gΪFe��OH��
3�����ȷֽ�����Fe
2O
3��n[Fe��OH��
3]=
=0.1mol������FeԪ���غ�n��Fe
2O
3��=
=0.05mol����m��Fe
2O
3��=0.05mol��160g/mol=8.0g��
�ʴ�Ϊ��8.0��
��3������FeԪ���غ�n��FeCl
3��=n[Fe��OH��
3]=0.1mol����c��FeCl
3��=
=1mol/L��
�ʴ�Ϊ��1��
��4���������ʱΪ26.3g��Ϊ������������������������֮�ͣ���������������Ϊ26.3g-10.7g=15.6g���������������ʵ���Ϊ
=0.2mol������AlԪ���غ�n��AlCl
3��=0.2mol��
��c��AlCl
3��=
=2mol/L��
�ʴ�Ϊ��2��
��5������550mL NaOH��Һ��ʱ����Һ������ΪNaCl��NaAlO
2�������������غ�n��NaCl��=3n��FeCl
3��+3n��AlCl
3��=3��0.1mol+3��0.2mol=0.9mol������AlԪ���غ�n��NaAlO
2��=n��AlCl
3��=0.2mol�������������غ�n��NaOH��=n��NaCl��+n��NaAlO
2��=0.9mol+0.2mol=1.1mol����c��NaOH��=
=2mol/L��
�ʴ�Ϊ��2��
�����������Ի�ѧ��Ӧͼ����ʽ���������ӷ�Ӧ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ���η����ķ�ӦΪ���ؼ���ע�������غ㷨�ڻ�ѧ�����е�Ӧ�÷���������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������ѧ����������





 ��R��R�������
��R��R���Ϊ����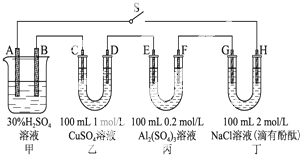 ��ͼʾ���ĸ������зֱ�ʢ�в�ͬ����Һ����A��B�⣬����缫��Ϊʯī�缫����ΪǦ���أ��乤��ԭ��Ϊ��Pb+PbO2+2H2SO4
��ͼʾ���ĸ������зֱ�ʢ�в�ͬ����Һ����A��B�⣬����缫��Ϊʯī�缫����ΪǦ���أ��乤��ԭ��Ϊ��Pb+PbO2+2H2SO4