
����22�֣�ijʵ��С��������װ�ý������µ�ʵ�顣
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
�� ��
ʵ��С���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ����ַ�Ӧ���ܼ������У�˵���÷�Ӧ�� �� ���� ��Ӧ��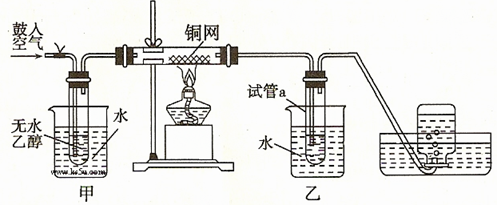
��2����������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
��3����Ӧ����һ��ʱ������ԇ��a�����ռ�����ͬ�����ʣ���ô�ռ������л����ǣ�����д��ṹ��ʽ�� ������ƿ���ռ��������������Ҫ�ɷ��� ������д����ķ���ʽ��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��ʵ��С���ͬѧ��Ϊ������Ǵ��ڸ��������ᡣ��ȥ�����ʣ�����ʹ�ó����г�����һ�ֻ����������Ϊ  ������д��ѧʽ�����ʽ��
������д��ѧʽ�����ʽ��
��5����д������ʵ���漰���л����Ҵ������������е�һ�־���Ӧ�ã� ��
��6����ʵ���Ŀ���ǣ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���22�֣�
I.ʵ����0.01mol/L��KMnO4��������Һ��0.1mol/L��H2C2O4��Һ�������Ϻ�Ӧ���ʦ�[mol/(L �� s)]�뷴Ӧʱ��t��s���Ĺ�ϵ��ͼ��ʾ���ش��������⣺
��1���÷�Ӧ�Ļ�ѧ����ʽ��
��2��0��t2ʱ����ڷ�Ӧ���������ԭ���ǣ� ��
��3��t2��tʱ����ڷ�Ӧ���ʼ�С��ԭ���ǣ� ��
��4��ͼ����Ӱ���֡��������ʾt1��t3ʱ���� ��
����Mn2+���ʵ���Ũ�ȵ����� B��Mn2+���ʵ���������
C��SO42-���ʵ���Ũ�� D��MnO4-���ʵ���Ũ�ȵļ�С
II. Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ����������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��1�����Է���������ͼ�ɹ۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
��2����������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������
��3������0.01mol MnO2��ĩ��60mL H2O2��Һ�У��ڱ�״���·ų����������ʱ��Ĺ�ϵ��ͼ��ʾ����ų�����������ΪV mL��
�ٷų�V/3 mL����ʱ����ʱ��Ϊ min��
�� ��H2O2��Һ��Ũ��Ϊ
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ > > >
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
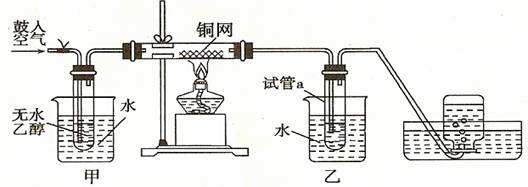
����14�֣�ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
�� ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�� ��Ӧ��
��2����������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
��3����Ӧ����һ��ʱ������a�����ռ�����ͬ�����ʣ������� ������ƿ���ռ������������Ҫ�ɷ��� ��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ����
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ�� ��������������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��һ6���¿���ѧ���� ���ͣ�ʵ����
����14�֣�ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
�� ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�� ��Ӧ��
��2����������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
��3����Ӧ����һ��ʱ������a�����ռ�����ͬ�����ʣ������� ������ƿ���ռ������������Ҫ�ɷ��� ��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ����
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ�� ��������������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����14�֣�ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
�� ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�� ��Ӧ��
��2����������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
��3����Ӧ����һ��ʱ������a�����ռ�����ͬ�����ʣ������� ������ƿ���ռ������������Ҫ�ɷ��� ��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ����
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ�� ��������������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com