
|
һ����������ȫȼ�գ������ɵ�����ͨ������ܣ����������7.2 g����ͨ��ʯ��ˮ��ʯ��ˮ����17.6 g�����ⶨ���������ܶ�����ͬ״����CO�ܶ���ȣ�����˵������ȷ���ǣ� | |
| [����] | |
A�� |
����һ����ʹ���Ը��������Һ��ɫ |
B�� |
������������ӳ������ĵ�n(Br2)������ӳɲ�������������ȫȡ��������n(Br2)�� |
C�� |
����ȼ��ʱ�������������к��� |
D�� |
��������ˮ���Ĵ���� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��101��Уͬ����ϰ����һ��ѧ��ɽ����ѧ���������� ³�̰� ���ͣ�022
ij��A��0.2 mol����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
(1)��A�ķ���ʽΪ________��
(2)��ȡһ����������ȫȼ�պ�����CO2��H2O��0.3 mol������________g���μ��˷�Ӧ��ȼ��ʱ���ı�������������Ϊ________��
(3)����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽΪ________��
(4)����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ________��
(5)����A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ����________��ͬ���칹�壮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��B�ķ���ʽΪ__________��
��2��д��C�Ľṹ��ʽ__________��
��3��A�ķ���ʽΪ__________��A�Ľṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��������У�߶���ѧ�ڵ�����������ѧ�Ծ��������棩 ���ͣ������
��.�л���A����Ϊ����B�����е�������ԭ�ӱ���C�������������ȡ�����õ��ġ�����֪����A��Br2��CCl4��Һ����ɫ����һ�ȴ���ֻ��һ�֡���һ������B��ȫȼ�գ�������n��CO2��:n��H2O����2:1����26<M��B��<78������CΪ����������ͨ���������̬����ͬ���칹�岻����2�֣�����������3�֡��Իش��������⣺
��1����B�����ʽΪ______������ʽΪ______��
��2����C������Ϊ ��
��3��A�ķ���ʽΪ________��
��.1,2-��������������Ϳ����������Ӽ�,������������ɫҺ��,�ܶ���2.18��/����3,�е�131.4��,�۵�9.79��,������ˮ,�����ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1,2-�������顣���з�Һ©������ƿa�зֱ�װ��Ũ������Ҵ���Һ���Թ�d��װ��Ũ�壨���渲������ˮ��������д���пհף�
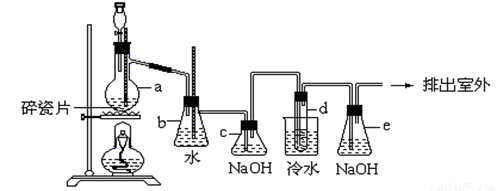
��1����д����ƿa�з����Ļ�ѧ��Ӧ����ʽ�� ��
��2����ȫƿb���Է�ֹ����,�����Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е����� ��
��3��cװ����NaOH��Һ�������� ��eװ����NaOH��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com