
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+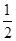 O2(g) ="==" Cu2O(s)
¦¤H=£169kJ”¤mol-1£¬
O2(g) ="==" Cu2O(s)
¦¤H=£169kJ”¤mol-1£¬
C(s)+ 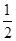 O2(g) ="==" CO(g)
¦¤H=£110.5kJ”¤mol-1£¬
O2(g) ="==" CO(g)
¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)===2 CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
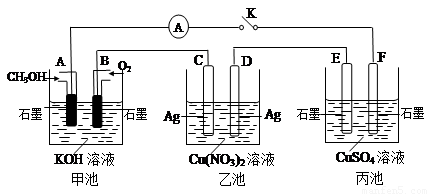
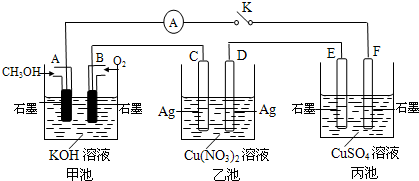
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
A£®Cu B£®CuO C£®Cu(OH)2 D£®Cu2(OH)2CO3
£Ø1£©C(s) + 2CuO (s)= Cu2O(s) + CO(g)  H =+34.5kJ”¤mol.-1£Ø3·Ö£©
H =+34.5kJ”¤mol.-1£Ø3·Ö£©
£Ø2£©¢ŁŌµē³Ų £Ø1·Ö£© CH3OH + 8OH££6e£ = CO32£+ 6H2O£Ø2·Ö£©
¢ŚŅõ¼«£Ø1·Ö£© 2CuSO4+ 2H2O 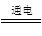 2H2SO4 + 2Cu + O2”ü£Ø2·Ö£©
2H2SO4 + 2Cu + O2”ü£Ø2·Ö£©
¢Ū560£Ø2·Ö£©
¢ÜA£Ø2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
C(s) + 2CuO (s)= Cu2O(s) + CO(g)  H =a£¬
H =a£¬ H µÄ¼ĘĖćČēĻĀ£¬C(s)+
H µÄ¼ĘĖćČēĻĀ£¬C(s)+ 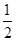 O2(g) ="==" CO(g) ¼õČ„2Cu(s)+ O2(g)===2 CuO(s) ŌŁ¼ÓÉĻ2Cu(s)+
O2(g) ="==" CO(g) ¼õČ„2Cu(s)+ O2(g)===2 CuO(s) ŌŁ¼ÓÉĻ2Cu(s)+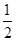 O2(g) ="==" Cu2O(s) æÉŅŌµĆµ½C(s) + 2CuO (s)= Cu2O(s) + CO(g) £¬ĖłŅŌ
O2(g) ="==" Cu2O(s) æÉŅŌµĆµ½C(s) + 2CuO (s)= Cu2O(s) + CO(g) £¬ĖłŅŌ H
=-110.5+314-169=+34.5kJ”¤mol.-1£¬ĖłŅŌ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
H
=-110.5+314-169=+34.5kJ”¤mol.-1£¬ĖłŅŌ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
C(s) + 2CuO (s)= Cu2O(s) + CO(g)  H =+34.5kJ”¤mol.-1£»
H =+34.5kJ”¤mol.-1£»
£Ø1£©ÓÉĶ¼æÉŅŌÖŖµĄ£¬¼×³ŲĪŖŌµē³Ų£¬Aµē¼«ĪŖøŗ¼«£¬øƵē¼«·“Ó¦Ź½ĪŖCH3OH
+ 8OH££6e£ = CO32£+ 6H2O£»£Ø2£©±ū³ŲÖŠFµē¼«Óėµē³ŲµÄøŗ¼«Į¬½Ó£¬ĖłŅŌFµē¼«ĪŖŅõ¼«£¬±ūµē³ŲĪŖµē½ā³Ų£¬øƵē³ŲµÄ×ܵķ“Ó¦Ź½ĪŖ2CuSO4+ 2H2O 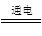 2H2SO4 + 2Cu + O2”ü£»£Ø3£©C¼«ÖŠ£¬AgČܽā£¬ĖłŅŌµ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼“ĶعżµÄµē×ÓŹżĪŖ10.8/108=0.1mol£¬ĖłŅŌBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»ż=0.1/4”Į22.4”Į1000=560mL£»£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ŅŅ³ŲŌŚµē½āµÄ¹ż³ĢÖŠŅżČėĮĖŅųĄė×Ó£¬ÓŠŅ»²æ·ÖĶĄė×ÓŌŚµē¼«ÉĻĪö³ö£¬ĖłŅŌ¼ÓČėCuæÉŅŌ»Öø“µ½ŌĄ“µÄÅØ¶Č£¬¹Ź±¾ĢāµÄ“š°øŃ”ŌńA”£
2H2SO4 + 2Cu + O2”ü£»£Ø3£©C¼«ÖŠ£¬AgČܽā£¬ĖłŅŌµ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼“ĶعżµÄµē×ÓŹżĪŖ10.8/108=0.1mol£¬ĖłŅŌBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»ż=0.1/4”Į22.4”Į1000=560mL£»£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ŅŅ³ŲŌŚµē½āµÄ¹ż³ĢÖŠŅżČėĮĖŅųĄė×Ó£¬ÓŠŅ»²æ·ÖĶĄė×ÓŌŚµē¼«ÉĻĪö³ö£¬ĖłŅŌ¼ÓČėCuæÉŅŌ»Öø“µ½ŌĄ“µÄÅØ¶Č£¬¹Ź±¾ĢāµÄ“š°øŃ”ŌńA”£
æ¼µć£ŗČČ»Æѧ·½³ĢŹ½µÄŹéŠ“”¢Ōµē³Ų”¢µē½ā³Ų
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖČČ»Æѧ·½³ĢŹ½µÄŹéŠ“”¢Ōµē³Ų”¢µē½ā³Ų£¬ÕāŠ©æ¼µćŹĒøßæ¼æ¼²éµÄÖŲµćŗĶÄŃµć£¬±¾ĢāÓŠŅ»¶ØµÄ×ŪŗĻŠŌ£¬ÄѶȏŹÖŠ”£


 ×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ą³ĪߏŠµŚŅ»ÖŠŃ§ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+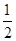 O2(g) ="==" Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
O2(g) ="==" Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
C(s)+ 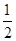 O2(g) ="==" CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
O2(g) ="==" CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)===2 CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
| A£®Cu | B£®CuO | C£®Cu(OH)2 | D£®Cu2(OH)2CO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”ÕŠÉś·ĀÕę¾ķĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©ÄÉĆ×¼¶Cu2 O ·ŪÄ©,ÓÉÓŚĮæ×ӳߓēŠ§Ó¦,Ęä¾ßÓŠĢŲŹāµÄ¹āѧ”¢µēѧ¼°¹āµē»ÆѧŠŌÖŹ,ŌŚĢ«Ńōµē³Ų”¢“«øŠĘ÷”¢³¬µ¼Ģ唢ÖĘĒāŗĶµēÖĀ±äÉ«”¢»·¾³ÖŠ“¦ĄķÓŠ»śĪŪČ¾ĪļµČ·½ĆęÓŠ×ÅĒ±ŌŚµÄÓ¦ÓĆ”£
¢ń£®ÄÉĆ×Ńõ»ÆŃĒĶµÄÖʱø
£Ø1£©ĖÄÖÖÖĘČ”Cu2OµÄ·½·ØČēĻĀ£ŗ
¢Ł»š·Ø»¹Ō”£ÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuO£»
¢Ś×īŠĀŹµŃéŃŠ¾æÓĆėĀ£ØN2H4£©»¹ŌŠĀÖĘCu(OH)2æÉÖʱøÄÉĆ×¼¶Cu2O£¬Ķ¬Ź±·Å³öN2”£
ŅŃÖŖ£ŗN2H4(l)+O2(g) N2(g)+2H2O(l)
”÷H=-a
kJ/mol
N2(g)+2H2O(l)
”÷H=-a
kJ/mol
Cu(OH)2(s) CuO(s)+H2O(l) ”÷H=b kJ/mol
CuO(s)+H2O(l) ”÷H=b kJ/mol
4CuO(s) 2Cu2O(s)+O2(g)
”÷H=c
kJ/mol
2Cu2O(s)+O2(g)
”÷H=c
kJ/mol
ŌņøĆ·½·ØÖʱøCu2OµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
¢Ū¹¤ŅµÖŠÖ÷ŅŖ²ÉÓƵē½ā·Ø£ŗÓĆĶŗĶīŃ×÷µē¼«£¬µē½āĀČ»ÆÄĘŗĶĒāŃõ»ÆÄʵĻģŗĻČÜŅŗ£¬µē½ā×Ü·½³ĢŹ½ĪŖ£ŗ2Cu+H2O Cu2O+H2”ü£¬ŌņŃō¼«·“Ó¦Ź½ĪŖ£ŗ
ӣ
Cu2O+H2”ü£¬ŌņŃō¼«·“Ó¦Ź½ĪŖ£ŗ
ӣ
¢Ü»¹æɲÉÓĆNa2SO3»¹ŌCuSO4·Ø£ŗ½«Na2SO3 ŗĶCuSO4¼ÓČėČܽā²ŪÖŠ£¬ÖĘ³ÉŅ»¶ØÅØ¶ČµÄČÜŅŗ£¬ĶØČėÕōĘų¼ÓČČ£¬ÓŚ100”ę~104”ę¼ä·“Ó¦¼“æÉÖʵƔ£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
¢ņ£®ÄÉĆ×Ńõ»ÆŃĒĶµÄÓ¦ÓĆ
£Ø2£©ÓĆÖʵƵÄCu2O½ųŠŠ“߻ƷֽāĖ®µÄŹµŃé
¢ŁŅ»¶ØĪĀ¶ČĻĀ£¬ŌŚ2 LĆܱÕČŻĘ÷ÖŠ¼ÓČėÄÉĆ×¼¶Cu2O²¢ĶØČė10. 0 molĖ®ÕōĘų£¬·¢Éś·“Ó¦£ŗ
2H2O(g)  2H2(g)£«O2(g)
”÷H£½£«484 kJ”¤mol£1
2H2(g)£«O2(g)
”÷H£½£«484 kJ”¤mol£1
T1ĪĀ¶ČĻĀ²»Ķ¬Ź±¶Ī²śÉśO2µÄĮæ¼ūĻĀ±ķ£ŗ
|
Ź±¼ä/min |
20 |
40 |
60 |
80 |
|
n(O2)/mol |
1.0 |
1.6 |
2.0 |
2.0 |
Ē°20 minµÄ·“Ó¦ĖŁĀŹ v(H2O)£½ £»øĆøĆĪĀ¶ČĻĀ£¬·“Ó¦µÄĘ½ŗā³£ŹżµÄ±ķ“ļŹ½K£½ £»ČōT2ĪĀ¶ČĻĀK£½0.4£¬T1 T2£ØĢī>”¢<”¢=£©
¢ŚÓŅĶ¼±ķŹ¾ŌŚt1Ź±æĢ“ļµ½Ę½ŗāŗó£¬Ö»øıäŅ»øöĢõ¼žÓÖ“ļµ½Ę½ŗāµÄ²»Ķ¬Ź±¶ĪÄŚ£¬H2µÄÅضČĖꏱ¼ä±ä»ÆµÄĒéæö£¬Ōņt1Ź±Ę½ŗāµÄŅĘ¶Æ·½ĻņĪŖ £¬t2Ź±øıäµÄĢõ¼žæÉÄÜĪŖ £»ČōŅŌK1”¢K2”¢K3·Ö±š±ķŹ¾t1Ź±æĢĘšøıäĢõ¼žµÄČżøöŹ±¼ä¶ĪÄŚµÄĘ½ŗā³£Źż£¬t3Ź±æĢƻӊ¼ÓČė»ņ¼õÉŁĢåĻµÖŠµÄČĪŗĪĪļÖŹ£¬ŌņK1”¢K2”¢K3µÄ¹ŲĻµĪŖ £»

¢ŪÓĆŅŌÉĻĖÄÖÖ·½·ØÖʵƵÄCu2OŌŚĘäĖüĢõ¼žĻąĶ¬ĻĀ·Ö±š¶ŌĖ®“߻Ʒֽā£¬²śÉśĒāĘųµÄĖŁĀŹvĖꏱ¼ät±ä»ÆČēĶ¼ĖłŹ¾”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ ”£

A£®·½·Ø¢Ū”¢¢ÜÖʵƵÄCu2O“߻Ɗ§ĀŹĻą¶Ō½Ļøß
B£®·½·Ø¢ÜÖʵƵÄCu2O×÷“߻ƼĮŹ±£¬Ė®µÄĘ½ŗā×Ŗ»ÆĀŹ×īøß
C£®“߻Ɗ§¹ūÓėCu2OæÅĮ£µÄ“ÖĻø”¢±ķĆę»īŠŌµČÓŠ
D£®Cu2O“ß»ÆĖ®·Ö½āŹ±£¬ŠčŅŖŹŹŅĖµÄĪĀ¶Č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£© ÄÉĆ×¼¶Cu2OÓÉÓŚ¾ßÓŠÓÅĮ¼µÄ“߻ƊŌÄܶųŹÜµ½¹Ų×¢”£ŅŃÖŖ£ŗ
2Cu(s)+![]() O2(g) === Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
O2(g) === Cu2O(s) ¦¤H=£169kJ”¤mol-1£¬
C(s)+ ![]() O2(g) === CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
O2(g) === CO(g) ¦¤H=£110.5kJ”¤mol-1£¬
2Cu(s)+ O2(g)=== CuO(s) ¦¤H=£314kJ”¤mol-1
Ōņ¹¤ŅµÉĻÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÖĘČ”Cu2OŗĶCOµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
£Ø2£©Ä³ŠĖȤŠ”×éµÄĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æÓŠ¹Ųµē»ÆѧµÄĪŹĢā(¼×”¢ŅŅ”¢±ūČż³ŲÖŠČÜÖŹ×ćĮæ)£¬µ±±ÕŗĻøĆ×°ÖƵĵē¼üKŹ±£¬¹Ū²ģµ½µēĮ÷¼ĘµÄÖøÕė·¢ÉśĮĖĘ«×Ŗ”£
 |
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×³ŲĪŖ (Ģī”°Ōµē³Ų”±”¢”°µē½ā³Ų”±»ņ ”°µē¶Ę³Ų”±)£¬Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©±ū³ŲÖŠFµē¼«ĪŖ (Ģī”°Õż¼«”±”¢”°øŗ¼«”±”¢”°Ņõ¼«”±»ņ”°Ńō¼«”±)£¬øĆ³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ
ӣ
£Ø3£©µ±³ŲÖŠC¼«ÖŹĮæ¼õĒį10.8 gŹ±£¬¼×³ŲÖŠBµē¼«ĄķĀŪÉĻĻūŗÄO2µÄĢå»żĪŖ mL(±ź×¼×“æö)”£
£Ø4£©Ņ»¶ĪŹ±¼äŗ󣬶ĻæŖµē¼üK£¬ĻĀĮŠĪļÖŹÄÜŹ¹ŅŅ³Ų»Öø“µ½·“Ó¦Ē°ÅØ¶ČµÄŹĒ (ĢīŃ”Ļī×ÖÄø)”£
A£®Cu B£®CuO C£®Cu(OH)2 D£®Cu2(OH)2CO3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com