
���й��ھ����˵����,����ȷ����
�پ��������ӳ���������������,���Է���;���Ǿ�����ԭ�������������,���Է���;
�ں��н��������ӵľ���һ�������Ӿ���;
�۹��ۼ��ɾ������Ӿ�����ۡ��е�;
��MgO��NaCl���־����У� MgO�ľ����ܽ�С���������۵�Ƚϵ�
�ݾ����Ǿ���ṹ�Ļ�����Ԫ,�����ڲ�������һ���������������ظ�����;
���御���ܲ�ȡ���ܶѻ���ʽ,��ʹ���ñȽ��ȶ�;
�߸ɱ�������,һ��CO2������Χ��12��CO2���ӽ���;CsCl��NaCl���������������ӵ���λ����ͬ
| A���٢ڢ� | B���ڢۢ� |
| C���ܢݢ� | D���ڢۢ� |
D
��������������پ��������ӳ���������������,���Է���;���Ǿ�����ԭ�������������,���Է��ԣ���ȷ���ں��н��������ӵľ��岻һ�������Ӿ��壬���Ȼ������н��������ӵ����ڷ��Ӿ��壬����; �۹��ۼ��ɾ���ԭ�Ӿ�����ۡ��е㣬���Ӿ�����۷е�ĸߵ����ɷ�֮���������Ĵ�С�����ģ�����; ��MgO��NaCl���־����У� MgO�ľ����ܽϴ��������۵�Ƚϸߣ����ݾ����Ǿ���ṹ�Ļ�����Ԫ,�����ڲ�������һ���������������ظ����У���ȷ; �����еľ�������϶�������еģ������ܲ�ȡ���ܶѻ���ʽ,��ʹ���ñȽ��ȶ�����ȷ; �߸ɱ�������,һ��CO2������Χ��12��CO2���ӽ���;CsCl��NaCl���������������ӵ���λ����ͬ��CsCl����λ����8��NaCl����λ����6����ȷ�����Դ�ѡD��
���㣺����Ծ�������ʡ����͡���������з�ʽ����λ�����ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
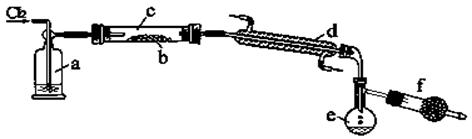
(10��) ������Ҫ��ش����У� ��BaCl2���ڱ�����NH4Cl����Na2SO4���ݸɱ�����Ƭ �������ʡ�
(1) �������ӻ��������__________(����ţ���ͬ)��������ֻ�����Ӽ���������________��
���ڹ��ۻ��������________��
(2) �ۻ�ʱ����Ҫ�ƻ���ѧ������__________���۵���͵���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CS2��S2Cl2���������Ҫ������ڹ�ҵ���й㷺����;�����ߵIJ����������£�
| | �۵�/�� | �е�/�� | �ܶ�/g��cm��3[��Դ | ˮ |
| CS2 | ��110.8 | 46.5 | 1.26 | ���� |
| S2Cl2 | ��76 | 138 | 1.75 | ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ѡ��3���ʽṹ�����ʡ���15�֣�
VIA�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ���������̬����VIA��Ԫ�صĻ�̨�����о�����������������Ҫ��;����ش��������⣺
��1��S���ʵij�����ʽΪS8���价״�ṹ����ͼ��ʾ��Sԭ�Ӳ��õĹ���ӻ���ʽ�� ��
��2��ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����
������Ҫ�����������O��S��Seԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ ��
��3��Seԭ������Ϊ �������M����ӵ��Ų�ʽΪ ��
��4��H2Se�����Ա�H2S ���ǿ��������������̬SeO3���ӵ����幹��
Ϊ ��SO32-���ӵ����幹��Ϊ ��
��5��H2SeO3��K1��K2�ֱ�Ϊ2.7x l0-3��2.5x l0-8��H2SeO4��һ��������ȫ���룬
K2Ϊ1.2X10-2������ݽṹ�����ʵĹ�ϵ���ͣ�
��H2SeO3��H2SeO4��һ������̶ȴ��ڵڶ��������ԭ��
��
�� H2SeO4�� H2SeO3����ǿ��ԭ��
��6��ZnS��ӫ���塢�����ϡ�Ϳ�ϡ����ϵ���ҵ��Ӧ�ù㷺������ZnS����ṹ����ͼ��ʾ���侧���߳�Ϊ540.0 pm���ܶ�Ϊ ����ʽ�����㣩��aλ��S2-������bλ��Zn2+����֮��ľ���Ϊ pm����ʾ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йؾ���������У�����ȷ����
| A���Ȼ��ƾ����У�ÿ��Na+��Χ����6��Cl- |
| B���Ȼ�菉����У�ÿ��CS+��Χ����8��Cl- |
| C�������ƾ����У�ÿ��F-��Χ����8��Ca2+��ÿ��Ca2+��Χ����8��F- |
| D���ɱ������У�ÿ��CO2������Χ����12��CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ������ַ�����һ������Դ��������ȼ������������Ҫ�ɷ��Ǽ�����ӵĽᾧˮ����(CH4��nH2O)�����γɹ����ǣ����ں��ز���Ĵ����л�����ȱ�������У�������ϸ�����л��ʷֽ⣬����γ�ʯ�ͺ���Ȼ��������������Ȼ��������ˮ�����У��ں��ĵ������ѹ���γ������Ʊ��������壬����ǡ���ȼ���������֡���ȼ�����ľ���������(����)
| A�����Ӿ��� | B�����Ӿ��� | C��ԭ�Ӿ��� | D���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������LawreceLiremore����ʵ���ң�LLNL����V.Lota.C.S.Yoo��Cynn�ɹ����ڸ�ѹ�½�CO2ת��Ϊ��������SiO2�ṹ��ԭ�Ӿ��壬���й���CO2��ԭ�Ӿ���˵����ȷ����
A��CO2��ԭ�Ӿ����д��ڷ��»�����ÿ1molCO2ԭ�Ӿ����к���2NA ��
��
B����һ�������£�CO2ԭ�Ӿ���ת��Ϊ���Ӿ���CO2�������仯
C���۵㣺���ʯ���Ȼ��ƣ�ԭ�Ӿ���CO2
D����CO2��ԭ�Ӿ����У�ÿ��Cԭ����Χ���4��Oԭ�ӣ�ÿ��Oԭ��������Cԭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и����У��������Ӿ��塢���Ӿ��塢ԭ�Ӿ����һ�ֵ��ǣ� ��
A�� B�� ���ʯ��
B�� ���ʯ��
C�� HF��SiC��Ar D�� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ˮ��״̬��������Һ����̬��в���̬��������Һ̬ˮ������ȴ��165 Kʱ�γɵģ�����̬��ˮ�̶���״�������ھ���ṹ�����ܶ�����ͨҺ̬ˮ���ܶ���ͬ���йز���̬ˮ��������ȷ���ǣ� ����
| A��ˮ��Һ̬��Ϊ����̬�������С |
| B��ˮ��Һ̬��Ϊ����̬��������� |
| C������̬��ˮ��һ������״̬ |
| D������̬ˮ�Ƿ��Ӿ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com