
¹¤ŅµĘ·“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClµČŌÓÖŹ£¬ĻĀĮŠĶ¼ÖŠµÄŅĒĘ÷×°ÖĆæÉÓĆĄ“²ā¶Ø“æ¼īÖŠµÄNa2CO3µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢Ł ”Ŗ æÕĘų£»¢Ś ”Ŗ ijČÜŅŗ£»¢Ū ”Ŗ “æ¼īѳʷ£»¢Ü ”Ŗ Ļ”H2SO4£»¢Ż ”Ŗ ÅØH2SO4£»¢Ž ”Ŗ ¼īŹÆ»Ņ£»¢ß ”Ŗ ¼īŹÆ»Ņ”£
ŹµŃé²½ÖčŹĒ£ŗ
¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£»
¢Ś×¼Č·³ĘĮæ×°ÓŠ¼īŹÆ»ŅµÄøÉŌļ¹ÜIµÄÖŹĮæ£ØÉčĪŖm1g£©£»
¢Ū×¼Č·³ĘĮæŅ»¶ØĮæµÄ“æ¼īµÄÖŹĮæ£ØÉčĪŖm2g£©£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
¢Ü“Ó·ÖŅŗĀ©¶·ÖŠ»ŗ»ŗµĪČėĻ”H2SO4£¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
¢Ż»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬Č»ŗó³ĘĮæøÉŌļ¹ÜIµÄ×ÜÖŹĮæ£ØÉčĪŖm3g£©”£
øł¾ŻÉĻŹöŹµŃ飬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¹ÄČėæÕĘųµÄÄæµÄŹĒ ”£
£Ø2£©×°ÖĆAÖŠŅŗĢå¢ŚÓ¦ĪŖ £¬Ęä×÷ÓĆŹĒ £¬Čē³·Č„×°ÖĆÖŠµÄAÖ±½ÓĻņ×°ÖĆBÖŠ»ŗ»ŗ¹ÄČėæÕĘų£¬²ā¶Ø½į¹ū½« £ØĢī”°Ę«“ó”±”¢”° Ę«Š””±»ņ”° ²»±ä”±”££©
£Ø3£©×°ÖĆCµÄ×÷ÓĆŹĒ £¬Čē¹ū³·Č„×°ÖĆC£¬Ōņ»įµ¼ÖĀŹµŃé½į¹ū £ØĢī”°Ę«“ó”±”¢”° Ę«Š””±»ņ”° ²»±ä”±”££©
£Ø4£©øÉŌļ¹Ü¢ņµÄ×÷ÓĆŹĒ ”£
£Ø5£©ÉĻŹöŹµŃé²Ł×÷µÄ¢ÜŗĶ¢Ż£¬¶¼ŅŖĒó»ŗ»ŗ½ųŠŠ£¬ĘäĄķÓÉŹĒ £¬Čē¹ūÕāĮ½²½²Ł×÷Ģ«æģŌņ»įµ¼ÖĀŹµŃé½į¹ū £ØĢī”°Ę«“ó”±”¢”° Ę«Š””±»ņ”° ²»±ä”±”££©
£Ø6£©øł¾ŻŹµŃ飬“æ¼īÖŠNa2CO3µÄÖŹĮæ·ÖŹż¼ĘĖćŹ½ĪŖ£ŗ ”£
 Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¹¤ŅµÉś²śµÄ“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClŌÓÖŹ£®Ä³Š£ŃŠ¾æŠŌѧĻ°»ī¶ÆŠ”×éĪŖĮĖ²ā¶Ø»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£¬ÄāŹ¹ÓĆČēĶ¼ŹµŃé×°ÖĆ£ØĖµĆ÷£ŗĮ¬½Ó¼×ŗĶŅŅµÄĻšĘ¤¹ÜÓŠĢś¼ŠæŲÖĘ£©£¬ĻČ²ā¶ØŅ»¶ØĮæµÄѳʷŗĶĖį·“Ó¦·Å³ö¶žŃõ»ÆĢ¼µÄÖŹĮ棬ŌŁ¼ĘĖć»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£®
¹¤ŅµÉś²śµÄ“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClŌÓÖŹ£®Ä³Š£ŃŠ¾æŠŌѧĻ°»ī¶ÆŠ”×éĪŖĮĖ²ā¶Ø»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£¬ÄāŹ¹ÓĆČēĶ¼ŹµŃé×°ÖĆ£ØĖµĆ÷£ŗĮ¬½Ó¼×ŗĶŅŅµÄĻšĘ¤¹ÜÓŠĢś¼ŠæŲÖĘ£©£¬ĻČ²ā¶ØŅ»¶ØĮæµÄѳʷŗĶĖį·“Ó¦·Å³ö¶žŃõ»ÆĢ¼µÄÖŹĮ棬ŌŁ¼ĘĖć»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£®| 53(w-m) |
| 22n |
| 53(w-m) |
| 22n |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ½Ģ²ÄĶźČ«½ā¶Į””ĖÕ½Ģ°ęæĪ±ź°ę””øßÖŠ»Æѧ””±ŲŠŽ1 ĖÕ½Ģ°ęæĪ±ź°ę ĢāŠĶ£ŗ058
¹¤ŅµĘ·“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClµČŌÓÖŹ£¬Ķ¼ÖŠµÄŅĒĘ÷×°ÖĆæÉÓĆĄ“²ā¶Ø“æ¼īÖŠNa2CO3µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢ŁæÕĘų£¬¢ŚÄ³ČÜŅŗ£¬¢Ū“æ¼īѳʷ£¬¢ÜĻ”H2SO4£¬¢ŻÅØH2SO4£¬¢Ž¼īŹÆ»Ņ£¬¢ß¼īŹÆ»Ņ£®
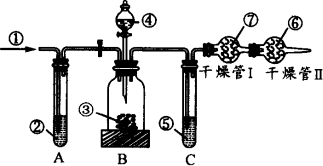
ŹµŃé²½ÖčŹĒ£ŗ
¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£»
¢Ś×¼Č·³ĘĮæŹ¢ÓŠ¼īŹÆ»ŅµÄøÉŌļ¹Ü¢ńµÄÖŹĮæ(ÉčĪŖm1 g)£»
¢Ū×¼Č·³ĘĮæŅ»¶ØĮæµÄ“æ¼īµÄÖŹĮæ(ÉčĪŖm2 g)£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
¢Ü“Ó·ÖŅŗĀ©¶·ÖŠ»ŗ»ŗµĪČėĻ”H2SO4£¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
¢Ż»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬Č»ŗó³ĘĮæøÉŌļ¹Ü¢ńµÄ×ÜÖŹĮæ(ÉčĪŖm3 g)£®
øł¾ŻÉĻŹöŹµŃ飬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¹ÄČėæÕĘųµÄÄæµÄŹĒ________£®
(2)×°ÖĆAÖŠŅŗĢå¢ŚÓ¦ĪŖ________£¬Ęä×÷ÓĆŹĒ________£®Čē³·Č„×°ÖĆA£¬Ö±½ÓĻņ×°ÖĆBÖŠ»ŗ»ŗ¹ÄČėæÕĘų£¬²ā¶Ø½į¹ū½«________£®(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)
(3)×°ÖĆCµÄ×÷ÓĆŹĒ________£¬Čē¹ū³·Č„×°ÖĆC£¬Ōņ»įµ¼ÖĀŹµŃé½į¹ū________£®(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)
(4)øÉŌļ¹Ü¢ņµÄ×÷ÓĆŹĒ________£®
(5)ÉĻŹöŹµŃéµÄ²Ł×÷¢ÜŗĶ¢Ż£¬¶¼ŅŖĒó»ŗ»ŗ½ųŠŠ£¬ĘäĄķÓÉŹĒ________£¬Čē¹ūÕāĮ½²½²Ł×÷Ģ«æģ£¬Ōņ»įµ¼ÖĀŹµŃé²ā¶Ø½į¹ū________£®(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)
(6)øł¾ŻŹµŃ飬“æ¼īÖŠNa2CO3µÄÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ058
¹¤ŅµĘ·“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClµČŌÓÖŹ£¬Ķ¼ÖŠµÄŅĒĘ÷×°ÖĆæÉÓĆĄ“²ā¶Ø“æ¼īÖŠ![]() µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢ŁæÕĘų£¬¢ŚÄ³ČÜŅŗ£¬¢Ū“æ¼īѳʷ£¬¢ÜĻ”
µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢ŁæÕĘų£¬¢ŚÄ³ČÜŅŗ£¬¢Ū“æ¼īѳʷ£¬¢ÜĻ” £¬¢ŻÅØ
£¬¢ŻÅØ £¬¢Ž¼īŹÆ»Ņ£¬¢ß¼īŹÆ»Ņ£®
£¬¢Ž¼īŹÆ»Ņ£¬¢ß¼īŹÆ»Ņ£®

ŹµŃé²½ÖčŹĒ£ŗ
¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£»
¢Ś×¼Č·³ĘĮæŹ¢ÓŠ¼īŹÆ»ŅøÉŌļ¹Ü¢ńµÄÖŹĮæ(ÉčĪŖ )£»
)£»
¢Ū×¼Č·³ĘĮæŅ»¶ØĮæµÄ“æ¼īµÄÖŹĮæ(ÉčĪŖ )£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
)£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
¢Ü“Ó·ÖŅŗĀ©¶·ÖŠ»ŗµĪČėĻ”![]() £¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
£¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
¢Ż»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬Č»ŗó³ĘĮæøÉŌļ¹Ü¢ńµÄ×ÜÖŹĮæ(ÉčĪŖ )£®
)£®
øł¾ŻÉĻŹöŹµŃ飬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¹ÄČėæÕĘųµÄÄæµÄŹĒ___________________________________£®
(2)×°ÖĆAÖŠŅŗĢå¢ŚÓ¦ĪŖ_________£¬Ęä×÷ÓĆŹĒ__________£¬Čē³·Č„×°ÖĆA£¬Ö±½ÓĻņ×°ÖĆBÖŠ»ŗ»ŗ¹ÄČėæÕĘų£¬²ā¶Ø½į¹ū½«_________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(3)×°ÖĆCµÄ×÷ÓĆŹĒ_____£¬Čē¹ū³·Č„×°ÖĆC£¬Ōņ»įµ¼ÖĀŹµŃé½į¹ū________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(4)øÉŌļ¹Ü¢ņµÄ×÷ÓĆŹĒ____________________________________£®
(5)ÉĻŹöŹµŃéµÄ²Ł×÷¢ÜŗĶ¢Ż£¬¶¼ŅŖĒó»ŗ»ŗ½ųŠŠ£¬ĘäĄķÓÉŹĒ________£¬Čē¹ūÕāĮ½²½²Ł×÷Ģ«æģ£¬Ōņ»įµ¼ÖĀŹµŃé²ā¶Ø½į¹ū_______(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(6)øł¾ŻŹµŃ飬“æ¼īÖŠ![]() µÄÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ__________£®
µÄÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ__________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ058
¹¤ŅµĘ·“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClµČŌÓÖŹ£¬Ķ¼ÖŠµÄŅĒĘ÷×°ÖĆæÉÓĆĄ“²ā¶Ø“æ¼īÖŠ![]() µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢ŁæÕĘų£¬¢ŚÄ³ČÜŅŗ£¬¢Ū“æ¼īѳʷ£¬¢ÜĻ”
µÄÖŹĮæ·ÖŹż£¬Ķ¼ÖŠ±źŗÅ£ŗ¢ŁæÕĘų£¬¢ŚÄ³ČÜŅŗ£¬¢Ū“æ¼īѳʷ£¬¢ÜĻ” £¬¢ŻÅØ
£¬¢ŻÅØ £¬¢Ž¼īŹÆ»Ņ£¬¢ß¼īŹÆ»Ņ£®
£¬¢Ž¼īŹÆ»Ņ£¬¢ß¼īŹÆ»Ņ£®

ŹµŃé²½ÖčŹĒ£ŗ
¢Ł¼ģ²é×°ÖƵÄĘųĆÜŠŌ£»
¢Ś×¼Č·³ĘĮæŹ¢ÓŠ¼īŹÆ»ŅøÉŌļ¹Ü¢ńµÄÖŹĮæ(ÉčĪŖ )£»
)£»
¢Ū×¼Č·³ĘĮæŅ»¶ØĮæµÄ“æ¼īµÄÖŹĮæ(ÉčĪŖ )£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
)£¬²¢½«Ęä·ÅČė¹ćæŚĘæÖŠ£»
¢Ü“Ó·ÖŅŗĀ©¶·ÖŠ»ŗµĪČėĻ”![]() £¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
£¬ÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
¢Ż»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬Č»ŗó³ĘĮæøÉŌļ¹Ü¢ńµÄ×ÜÖŹĮæ(ÉčĪŖ )£®
)£®
øł¾ŻÉĻŹöŹµŃ飬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¹ÄČėæÕĘųµÄÄæµÄŹĒ___________________________________£®
(2)×°ÖĆAÖŠŅŗĢå¢ŚÓ¦ĪŖ_________£¬Ęä×÷ÓĆŹĒ__________£¬Čē³·Č„×°ÖĆA£¬Ö±½ÓĻņ×°ÖĆBÖŠ»ŗ»ŗ¹ÄČėæÕĘų£¬²ā¶Ø½į¹ū½«_________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(3)×°ÖĆCµÄ×÷ÓĆŹĒ_____£¬Čē¹ū³·Č„×°ÖĆC£¬Ōņ»įµ¼ÖĀŹµŃé½į¹ū________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(4)øÉŌļ¹Ü¢ņµÄ×÷ÓĆŹĒ____________________________________£®
(5)ÉĻŹöŹµŃéµÄ²Ł×÷¢ÜŗĶ¢Ż£¬¶¼ŅŖĒó»ŗ»ŗ½ųŠŠ£¬ĘäĄķÓÉŹĒ________£¬Čē¹ūÕāĮ½²½²Ł×÷Ģ«æģ£¬Ōņ»įµ¼ÖĀŹµŃé²ā¶Ø½į¹ū_______(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£®
(6)øł¾ŻŹµŃ飬“æ¼īÖŠ![]() µÄÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ__________£®
µÄÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ__________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com