



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
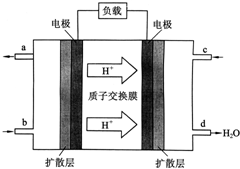 �й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѣ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��
�й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѣ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����Na2S��Һ�м�ˮϡ�ͣ�
| ||
| B��CH3COONH4��Һ�У�c��NH4+��+c��NH3?H2O��=c��CH3COOH��+c��CH3COO-�� | ||
| C��HCO3- ˮ������ӷ���ʽΪ��HCO3-+H2O?CO32-+H3O+ | ||
| D������ȥCuCl2��Һ�е�����FeCl2��Ӧ����CuO���ٹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1g��Ͷ��100gˮ�У���Һ��������Ϊ100g |
| B��1g���汻�����Ľ�����Ͷ��100gˮ�У���Һ��������Ϊ101g |
| C��1g������Ͷ��100gˮ�У���Һ��������Ϊ101g |
| D��1g��������Ͷ��100gˮ�У���Һ��������Ϊ101g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com