
����ZnO�������ϡ�Ϳ�Ϲ�ҵ������ҪӦ�ã�һ���ɴ�ZnO(��FeO��CuO)�Ʊ�����ZnO����������(��֪����ʽ̼��п�����տ��Ƶû���ZnO)�� 
��֪�������������������������ʱ��pH�����
��ش��������⣺
(1)����A��H2O2������Ӧ�����ӷ���ʽ�� ���ò����������ҺpH�ķ�Χ�� ��
(2)A��Һ����Ҫ���е������� ��
(3)��ʽ̼��п�������Ƶû���ZnO�ķ�Ӧ��H��0���÷�Ӧ���Է����е�ԭ���Ǧ�S (ѡ�=��������������)0��
(4)����������ķ�ˮpH=8����ʱZn2+��Ũ��Ϊ mg/L(�����£�Ksp��Zn(OH)2��=1.2��10-17)��
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ˮ�к���������[Au(CN)2]������������CN���ж�����CN����H���������HCNʱ�����Ը�ǿ���ش��������⡣
(1)�綾��HCN��ˮ��Һ���Ժ�����д�������ķ���ʽ�� ��
(2)���������̼��ĵ��뷽ʽ���ƣ�[Au(CN)2]��Ҳ�������������룬��һ�����뷽��ʽΪ ��
(3)�������ַ�ˮ���ڼ��������£�����NaClO��CN������ΪCO32-��N2�������ӷ���ʽΪ �������������£�ClO��Ҳ������CN������ʵ�ʴ�����ˮʱȴ�������������½��е���Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��1�������£�ȡpH��2������ʹ�����Һ��100 mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���________���A����B�������������вμӷ�Ӧ��Zn������Ϊm1��������Һ�вμӷ�Ӧ��Zn������Ϊm2����m1________m2��ѡ���������������������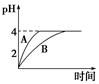
��2����֪������Cu��OH��2��Ksp��2��10��20����֪������ijCuSO4��Һ��c��Cu2������0.02 mol��L��1�����Ҫ����Cu��OH��2��������Ӧ������ҺpH����________��Ҫʹ0.2 mol��L��1��CuSO4��Һ��Cu2��������Ϊ��ȫ��ʹCu2��Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ���NaOH��Һ��ʹ��ҺpHΪ________��
��3��10 ��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
| �¶�/�� | 10 | 20 | 30 | ������к���ȴ��50 �� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����25��ʱ����Ũ�Ⱦ�Ϊ0.01 mol��L��1��MgCl2��AlCl3�����Һ����μ��백ˮ��������________����(�ѧʽ)�����ɸó��������ӷ���ʽΪ_____________________��(��֪25��ʱKsp[Mg(OH)2]��1.8��10��11��Ksp[Al(OH)3]��3��10��34��)
��2��ij�¶�(t��)ʱ�����0.01 mol��L��1��NaOH��Һ��pH��11���ڴ��¶��£���pH��2��H2SO4��ҺVaL��pH��12��NaOH��ҺVbL��ϣ������û��ҺΪ���ԣ���Va�UVb��________��
��3����25��ʱ����c mol��L��1�Ĵ�����Һ��0.02 mol��L��1NaOH��Һ�������Ϻ���Һ�պó����ԣ��ú�c�Ĵ���ʽ��ʾCH3COOH�ĵ��볣��Ka��________��
��4��(2013��ɽ���߿�)25��ʱ��H2SO3 HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��
HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��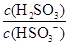 ��________(���������С�����䡱)��
��________(���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ��H2B=H����HB����HB��??H����B2�����ش��������⡣
��1��Na2B��Һ��________������ԡ��������ԡ����ԡ�����������________________�������ӷ���ʽ��ʾ����
��2����0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����________��
A��c��B2������c��HB������c��H2B����0.1 mol��L��1
B��c��Na������c��OH������c��H������c��HB����
C��c��Na������c��H������c��OH������c��HB������2c��B2����
D��c��Na������2c��B2������2c��HB����
��3����֪0.1 mol��L��1 NaHB��Һ��pH��2����0.1 mol��L��1 H2B��Һ�е������ӵ����ʵ���Ũ�ȿ���________0.11 mol��L��1�����������������������������_________________________________________________________________��
��4��0.1 mol��L��1 NaHB��Һ�и�������Ũ���ɴ�С��˳����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��
�����ᡢ�ڴ�����Һ��������������Һ�����Ȼ����Һ���ݴ������Һ�����������Һ�������������Һ���ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳���ǣ�����ţ�________��
��2���ܡ��ݡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�________��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ�ȹ�ϵ��ȷ����________��
| A��c��Na������c��Cl������c��OH������c��NH4+�� |
| B��c��Na������0.1 mol��L��1 |
| C��c��Na������c��NH4+����c��Cl������c��OH���� |
| D��c��H������c��OH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���100 mL 0.01 mol��L��1 HA��Һ����μ���0.02 mol��L��1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ���)��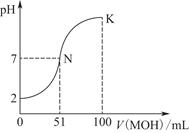
�ش��������⣺
(1)��ͼ����Ϣ��֪HAΪ________��(�ǿ��������)��������________________________________________________��
(2)������һ��Ũ�ȵ�MAϡ��Һ��pH��a����a________________________________________________________7
(�>����<������)�������ӷ���ʽ��ʾ��ԭ��Ϊ_____________________________________________________
��ʱ����Һ����ˮ�������c(OH��)��________��
(3)��д��K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��_________________________________________��
(4)K���Ӧ����Һ�У�c(M��)��c(MOH)________2c(A��)(�>����<������)������ʱ��Һ�У�pH��10����c(M��)��c(OH��)��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(H3PO3)�Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
(1)PCl3ˮ�����ȡ�����PCl3��3H2O===H3PO3��________��
(2)H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3 H����H2PO3����
H����H2PO3����
��ij�¶��£�0.10 mol��L��1��H3PO3��ҺpH��1.6������Һ��c(H��)��2.5��
10��2 mol��L��1������¶�����������ƽ���ƽ�ⳣ��K��д��������̡�(H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч����)
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH________7(�>����������<��)��
(3)���������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
(4)���Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
�������ĵ缫��ӦʽΪ________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
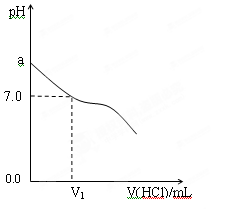
��1�������£���NH3��H2O������������ϣ�ʵ���������£�
| ��� | NH3��H2O | HCl | �����Һ��pHֵ |
| �� | c��NH3��H2O��=0.1mol��L-1 | c��HCl��=0.1mol��L-1 | pH=a |
| �� | NH3��H2O��pH=12 | HCl��pH=2 | pH=b |
| �� | c��NH3��H2O��="A" mol��L-1 | c��HCl��=0.1mol��L-1 | pH=c |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com