
 ČēĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©øł¾ŻĪļÖŹµÄĮæÅضČc=$\frac{1000¦Ń¦Ų}{M}$Ą“¼ĘĖć£»
£Ø2£©¢Łøł¾ŻČÜŅŗĻ”ŹĶ¶ØĀÉCÅØVÅØ=CĻ”VĻ”Ą“¼ĘĖć£»
¢Śøł¾ŻÅäÖĘ²½ÖčŹĒ¼ĘĖć”¢ĮæČ””¢Ļ”ŹĶ”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ”¢×°ĘæĄ“·ÖĪöŠčŅŖµÄŅĒĘ÷£»
¢Ūøł¾Żc=$\frac{n}{V}$²¢½įŗĻČÜÖŹµÄĪļÖŹµÄĮænŗĶČÜŅŗµÄĢå»żVµÄ±ä»ÆĄ“½ųŠŠĪó²ī·ÖĪö£»
£Ø3£©øł¾Żn=$\frac{V}{{V}_{m}}$¼ĘĖćĀČ»ÆĒāĘųĢåµÄĪļÖŹµÄĮ棬ŌŁøł¾Żm=nM¼ĘĖćHClµÄÖŹĮ棬øł¾Żm=¦ŃV¼ĘĖćĖ®µÄÖŹĮ棬½ų¶ų¼ĘĖćČÜŅŗµÄÖŹĮ棬øł¾ŻV=$\frac{m}{¦Ń}$¼ĘĖćČÜŅŗµÄĢå»ż£¬øł¾Żc=$\frac{n}{V}$¼ĘĖćøĆŃĪĖįµÄĪļÖŹµÄĮæÅØ¶Č£®
½ā“š ½ā£ŗ£Ø1£©ČÜŅŗµÄĪļÖŹµÄĮæÅضČc=$\frac{1000¦Ń¦Ų}{M}$=$\frac{1000”Į1.2”Į36.5%}{36.5}$=12mol/L£¬¹Ź“š°øĪŖ£ŗ12£»
£Ø2£©¢ŁÉčĖłŠčµÄÅØŃĪĖįµÄĢå»żĪŖVmL£¬øł¾ŻČÜŅŗĻ”ŹĶ¶ØĀÉCÅØVÅØ=CĻ”VĻ”æÉÖŖ£ŗ12mol/L”ĮVmL=0.7mol/L”Į250mL£¬½āµĆV=14.6mL£¬¹Ź“š°øĪŖ£ŗ14.6£»
¢Śøł¾ŻÅäÖĘ²½ÖčŹĒ¼ĘĖć”¢ĮæČ””¢Ļ”ŹĶ”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ”¢×°ĘææÉÖŖĖłŠčµÄŅĒĘ÷ÓŠĮæĶ²”¢ÉÕ±”¢²£Į§°ō”¢250mLČŻĮæĘæŗĶ½ŗĶ·µĪ¹Ü£¬¹Ź»¹Č±ÉŁ250mLČŻĮæĘ棬¹Ź“š°øĪŖ£ŗ250mLČŻĮæĘ棻
¢ŪA”¢ÓĆĮæĶ²ĮæČ”ÅØŃĪĖįŹ±ø©ŹÓ°¼ŅŗĆę£¬ŌņÅØŃĪĖįµÄĢå»żĘ«Š”£¬ÅäÖĘ³öµÄČÜŅŗµÄÅضČĘ«Š”£¬¹ŹAÕżČ·£»
B”¢Ī“»Öø“µ½ŹŅĪĀ¾Ķ½«ČÜŅŗ×¢ČėČŻĮæĘæ²¢½ųŠŠ¶ØČŻ£¬ŌņĄäČ“ŗóČÜŅŗĢå»żĘ«Š”£¬ÅضČĘ«øߣ¬¹ŹB“ķĪó£»
C”¢ČŻĮæĘæÓĆÕōĮóĖ®Ļ“ŗóĪ“øÉŌļ£¬¶ŌĖłÅäČÜŅŗµÄÅضČĪŽÓ°Ļģ£¬¹ŹC“ķĪó£»
D”¢¶ØČŻŹ±ŃöŹÓŅŗĆę£¬ŌņČÜŅŗĢå»żĘ«“ó£¬ÅضČĘ«Š”£¬¹ŹDÕżČ·£»
E”¢Ī“Ļ“µÓÉÕ±ŗĶ²£Į§°ō£¬Ōģ³ÉČÜÖŹµÄĖšŹ§£¬ŌņÅضČĘ«Š”£¬¹ŹEŃ”£®
¹ŹŃ”ADE£®
£Ø3£©HClµÄĪļÖŹµÄĮæĪŖ$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol£¬HClµÄÖŹĮæĪŖ$\frac{a}{22.4}$mol”Į36.5g/mol=$\frac{36.5a}{22.4}$g£¬1LĖ®µÄÖŹĮæĪŖ1000mL”Į1g/mL=1000g£¬¹ŹČÜŅŗµÄÖŹĮæĪŖ£Ø$\frac{36.5a}{22.4}$+1000£©g£¬ČÜŅŗµÄĢå»żĪŖ$\frac{£Ø\frac{36.5a}{22.4}+1000£©g}{1000dg/L}$=$\frac{36.5a+22400}{22400d}$L£¬¹ŹĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ$\frac{\frac{a}{22.4}L}{\frac{36.5a+22400}{22400d}L}$=$\frac{1000da}{36.5a+22400}$mol/L£¬
¹ŹŃ”d£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ¹ż³ĢÖŠµÄ¼ĘĖćŗĶĪó²ī·ÖĪö£¬ŹōÓŚ»ł“”ŠĶĢāÄ棬ÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
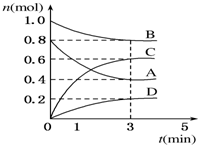 T”ꏱ£¬ŌŚČŻ»żĪŖ0.5LµÄĆܱÕČŻĘ÷ÖŠ·¢ÉśČē ĻĀ·“Ó¦£®mA£Øg£©+nB£Øg£©?pC£Øg£©+qD£Øs£©”÷H£¼0£Øm”¢n”¢p”¢qĪŖ×ī¼ņÕūŹż±Č£©£®A”¢B”¢C”¢DµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼ĖłŹ¾£®
T”ꏱ£¬ŌŚČŻ»żĪŖ0.5LµÄĆܱÕČŻĘ÷ÖŠ·¢ÉśČē ĻĀ·“Ó¦£®mA£Øg£©+nB£Øg£©?pC£Øg£©+qD£Øs£©”÷H£¼0£Øm”¢n”¢p”¢qĪŖ×ī¼ņÕūŹż±Č£©£®A”¢B”¢C”¢DµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×ĶéµÄ±ź×¼Č¼ÉÕČČĪŖ-890.3 kJ•mol-1£¬Ōņ¼×ĶéČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½æɱķŹ¾ĪŖCH4£Øg£©+2O2£Øg£©ØTCO2£Øg£©+2H2O£Øg£©”÷H=-890.3 kJ•mol-1 | |
| B£® | 500”ę”¢300 MPaĻĀ£¬½«0.5 mol N2ŗĶ1.5 mol H2ÖĆÓŚĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ӧɜ³ÉNH3£Øg£©£¬·ÅČČ19.3 kJ£¬ĘäČČ»Æѧ·½³ĢŹ½ĪŖN2£Øg£©+3H2£Øg£©$?_{500”ę”¢300MPa}^{“߻ƼĮ}$ 2NH3£Øg£©”÷H=-38.6kJ•mol-1 | |
| C£® | ĀČ»ÆĆ¾ČÜŅŗÓė°±Ė®·“Ó¦£ŗMg2++2OH-ØTMg£ØOH£©2”ż | |
| D£® | Ńõ»ÆĀĮČÜÓŚNaOHČÜŅŗ£ŗA12O3+2OH-ØT2AlO${\;}_{2}^{-}$+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
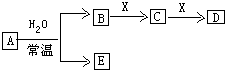 ÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄ֊ѧ³£¼ūĪŽ»śĪļA”¢B”¢C”¢D”¢E”¢X“ęŌŚČēĶ¼×Ŗ»Æ¹ŲĻµ£Ø²æ·ÖÉś³ÉĪļŗĶ·“Ó¦Ģõ¼žĀŌČ„£©£®
ÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄ֊ѧ³£¼ūĪŽ»śĪļA”¢B”¢C”¢D”¢E”¢X“ęŌŚČēĶ¼×Ŗ»Æ¹ŲĻµ£Ø²æ·ÖÉś³ÉĪļŗĶ·“Ó¦Ģõ¼žĀŌČ„£©£® £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģ¼ĖįÄĘ | B£® | ŅŗĢ¬µŖ | C£® | ŃĪĖį | D£® | ĒāŃõ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com