
��14�֣���ͼ��ʾʵ������û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã� ͼ��a��b�ǿɿ��Ƶĵ������С�

��1������A�������� ��ˮ����Ӧʢ�ŵ��� ���ձ��з�Ӧ�Ļ�ѧ����ʽ ��
��2�����ռ�����ʱ��Ӧ ���������ռ����ʱӦ ��������ο���a��b��
��3����Ҫ�Ƶñ�״���µ�Cl2 0.672L����������ҪMnO2�����ʵ����� mol��
��4��ʵ����Ҳ���ڲ����ȵ��������ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ���£�
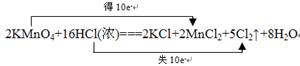
2KMnO4+16HCl(Ũ)===2KCl+2MnCl2+5Cl2��+8H2O
�÷�Ӧ�У��������� ������������ ������1mol���ӷ���ת��ʱ��
�������������Ϊ ����״����������˫���ű�ʾ��������ԭ��Ӧ��
2KMnO4+16HCl(Ũ)===2KCl+2MnCl2+5Cl2��+8H2O
��1����Һ©��������ʳ��ˮ��Cl2+2NaOH=NaCl+NaClO+H2O��
��2����a, �ر�b����b���ر�a�� ��3��0.03 mol��
��4���������� KMnO4������������Cl2��11.2L
��������
�����������1������A�����ƽз�Һ©����Ϊ�˽����������ܽ�ȣ�ˮ��Ӧ�÷ű���ʳ��ˮ���ձ���Ӧ�÷�NaOH��Һ���ն������������Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O��
��2���ռ�����ʱ��Ӧ�ô�a�ر�b���ռ���Ϻ�Ӧ�ùر�a����b��
��3�����ݷ�Ӧ��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O����֪�Ƶñ�״���µ�Cl2 0.672����������ҪMnO2�����ʵ�����0.03 mol��
MnCl2+Cl2��+2H2O����֪�Ƶñ�״���µ�Cl2 0.672����������ҪMnO2�����ʵ�����0.03 mol��
��4�����ݷ�Ӧ2KMnO4+16HCl(Ũ)===2KCl+2MnCl2+5Cl2��+8H2O����֪��������KMnO4������������Cl2����1mol���ӷ���ת��ʱ���������������Ϊ11.2L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ��ԭ��������и�һ��ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺

��1����Ũ������HCl�����ʵ���Ũ��Ϊ________mol/L��
ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.3 mol/Lϡ���
��2����ѧ����Ҫ��ȡ________ mL����Ũ����������ơ�
��3�����ƹ����У�����Ҫʹ���ձ�����Ͳ���������⣬����Ҫʹ�õ������ǣ���д���ƣ�
�� ��
��4������ʱ������ȷ�IJ���˳���ǣ�Ҫ������ĸ��ʾ��ÿ����ĸֻ����һ�Σ� __________��
A����30mL����ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ����������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�����ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�����ˮ��ֱ��Һ��ӽ�����1��2cm��
��5�������ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���________
A��ҡ�Ⱥ��ã�����Һ����ڿ��ߣ�������ˮ�����������
B����Һע������ƿǰû�лָ������¾ͽ��ж���
C������ʱ���ӿ���
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���ϸ���ѧ��һ��ѧ�����л�ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣���ͼ��ʾʵ������û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã�ͼ��a��b�ǿɿ��Ƶĵ������С�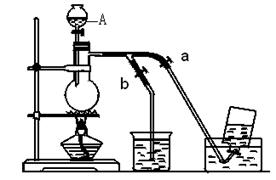
��1������A�������� ��ˮ����Ӧʢ�ŵ��� ���ձ��з�Ӧ�Ļ�ѧ����ʽ ��
��2�����ռ�����ʱ��Ӧ ���������ռ����ʱӦ ��������ο���a��b��
��3����Ҫ�Ƶñ�״���µ�Cl2 0.672L����������ҪMnO2�����ʵ����� mol��
��4��ʵ����Ҳ���ڲ����ȵ��������ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ���£�
2KMnO4+16HCl(Ũ)===2KCl+2MnCl2+5Cl2��+8H2O
�÷�Ӧ�У��������� ������������ ������1mol���ӷ���ת��ʱ��
�������������Ϊ ����״����������˫���ű�ʾ��������ԭ��Ӧ��
2KMnO4+16HCl(Ũ)===2KCl+2MnCl2+5Cl2��+8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����и߶���ѧ����ĩ��ѧ�������л�ѧ���� ���ͣ�ʵ����
���Ȼ�����S2Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������������ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2�����������ʣ�
| �������� | ɫ̬ | �ӷ��� | �۵� | �е� |
| ���ɫҺ�� | �ӷ� | ��76�� | 138�� | |
| ��ѧ���� | 300 ��������ȫ�ֽ� | |||
S2Cl2��Cl2 2SCl2 2SCl2 | ||||
| ��ˮ��Ӧ����SO2��S�Ȳ��� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����и߶���ѧ����ĩ��ѧ�������л�ѧ���� ���ͣ�ʵ����
���Ȼ�����S2Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������������ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2�����������ʣ�
|
�������� |
ɫ̬ |
�ӷ��� |
�۵� |
�е� |
|
���ɫҺ�� |
�ӷ� |
��76�� |
138�� |
|
|
��ѧ���� |
300 ��������ȫ�ֽ� |
|||
|
S2Cl2��Cl2 |
||||
|
��ˮ��Ӧ����SO2��S�Ȳ��� |
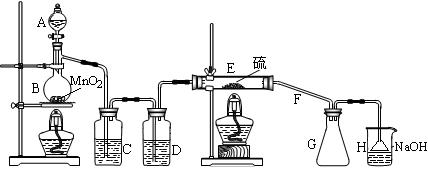
�����ڵ�����ͨ�Ը��������C12��������S2C12����ͼ��ʵ�����Ʊ�S2Cl2��װ�á�

��1������A�������� ������F���������������� ��
��2��B�з�Ӧ�����ӷ���ʽΪ ��
��3����ʼʵ�飬��Һ©���Ļ���������Ũ�������������������µΣ�������
Һ©������û�ж���������ΪӦ��ȡ�Ĵ�ʩ�� ��
��4��װ��C�е��Լ��� �����ȱ��Dװ�ã����ʵ���Ӱ���ǣ��û�ѧ����ʽ��ʾ�� ��
��5������ڼ���Eʱ�¶ȹ��ߣ���ʵ������Ӱ���� ��Ϊ�����S2C12�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶Ⱥ� ��
��6�����װ��H��Ŀ���� ���ձ��з�����Ӧ�����ӷ���ʽ
�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com