
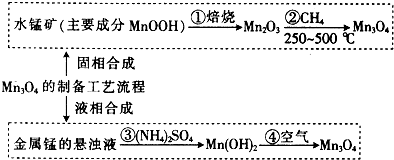
���� ���Ʊ����̿�֪��MnOOH����Mn2O3�����з���12Mn2O3+CH4$\frac{\underline{\;250-500��\;}}{\;}$8Mn3O4+CO2+2H2O��Mn3O4���̵ļ�̬������+2��+4����ɣ���д��2MnO•MnO2�������̵�����Һ������立�����ӦΪMn+2NH4++2H2O=Mn2++H2��+2NH3•H2O�����˳���Mn��OH��2��Ҫϴ�ӣ��ڿ����м�������Mn3O4���Դ������
��� �⣺���Ʊ����̿�֪��MnOOH����Mn2O3�����з���12Mn2O3+CH4$\frac{\underline{\;250-500��\;}}{\;}$8Mn3O4+CO2+2H2O��Mn3O4���̵ļ�̬������+2��+4����ɣ���д��2MnO•MnO2�������̵�����Һ������立�����ӦΪMn+2NH4++2H2O=Mn2++H2��+2NH3•H2O�����˳���Mn��OH��2��Ҫϴ�ӣ��ڿ����м�������Mn3O4��
��1����Mn3O4���̵ļ�̬������+2��+4����ɣ������������γɵı���ʽ2MnO•MnO2��MnO2•2MnO���ʴ�Ϊ��2MnO•MnO2��MnO2•2MnO��
��2��OΪ-2�ۣ�HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0��֪��MnOOH���̵ļ�̬Ϊ+3���ڵĻ�ѧ����ʽΪ12Mn2O3+CH4�T8Mn3O4+CO2+2H2O��
�ʴ�Ϊ��+3��12Mn2O3+CH4�T8Mn3O4+CO2+2H2O��
��3������NH4��2SO4����ˮʹ�̵�����Һ�����ԣ��漴�����ز������ݣ�����Ӧ�����ӷ���ʽ����ԭ��ΪMn+2NH4++2H2O=Mn2++H2��+2NH3•H2O�����˳���Mn��OH��2��Ҫϴ�ӣ���Ҫ˵��ϴ�ӳ����IJ�������Ϊ��������м�����ˮ��û��������ˮ��Ȼ�������ظ���������2-3�Σ�
�ʴ�Ϊ��Mn+2NH4++2H2O=Mn2++H2��+2NH3•H2O����������м�����ˮ��û��������ˮ��Ȼ�������ظ���������2-3�Σ�
��4����Mn+2NH4++2H2O=Mn2++H2��+2NH3•H2O��6Mn��OH��2+O2$\frac{\underline{\;\;��\;\;}}{\;}$2Mn3O4+6H2O��֪������3H2��Mn3O4��������ռ���672mL����״���£���H2���������Ͽ��Եõ�Mn3O4������Ϊ$\frac{0.672L}{22.4L/mol}$��$\frac{1}{3}$��229g/mol=2.29g���ʴ�Ϊ��2.29��
���� ���⿼�����ʵ��Ʊ�ʵ�飬Ϊ��Ƶ���㣬�����Ʊ������еķ�Ӧ�����ʵ����ʡ�ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ�鷽�� | ʵ��Ŀ�Ļ���� |
| A | ȡһ��Сľ�������뱥��������Һ�У������ʪ������ȡ�������ɺ����ھƾ������洦��ľ��δȼ�� | ֤�������ƿ���ľ�ķ���� |
| B | ��ȡ��δ֪Ũ������������Һ����ƿ�м���2mL��̪��Ȼ�������ȷ�ζ������һ��������룬��Һ�ɺ�ɫ��Ϊ��ɫ�Ұ���Ӳ��ָ� | ȷ�ж���֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һ�ĵζ����յ� |
| C | ��װ��ʯ��ʯ�ļ������շ������м���Ũ���ᣬ��������������ͨ�뱥��̼��������Һ����ͨ�뱽������Һ�У���������Һ�������� | ���ԣ����̼����� |
| D | ��ʢ�б��ӵ�Ũ��Һ���Թ�����μ���ϡ��ˮ���ߵα��� | ���ӵĶ��Լ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 molNaN3��������������Ϊ3NA | |
| B�� | ���³�ѹ�£�28 g��ϩ�����ЦҼ���м���Ŀ֮��Ϊ6NA | |
| C�� | ��״���£�22.4L C12ͨ�뵽����FeBr2��Һ�У���������Br-��ĿΪ2NA | |
| D�� | 500 mL 18 mol/L��H2SO4��Һ������Cu���ȣ�����ת����ĿΪ9NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �⻯������ȶ��ԣ�D��E | |
| B�� | ԭ�Ӱ뾶��A��B��C��D��E | |
| C�� | B��C�γɵĻ������в����ܺ����ۼ� | |
| D�� | ����������Ӧ��ˮ����������ǿ����E |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
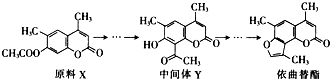
| A�� | X��Y��Ϊͬ���칹�� | |
| B�� | X��Y������ʹ����KMnO4��Һ��ɫ | |
| C�� | �ڹ��������£����������еı�������Cl2����ȡ����Ӧ | |
| D�� | �������������в����ͼ��������巢���ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
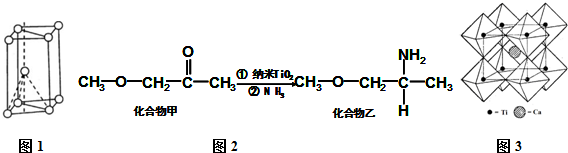
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | ���� | |
| A | �ٵμ�ϡHNO3 �ڵμ�BaCl2��Һ | ���������� ��ɫ���� | ԭ��Һһ����SO${\;}_{4}^{2-}$ |
| B | �μ�ϡ���� | �д������ݲ��� | ԭ��Һһ����CO${\;}_{3}^{2-}$ |
| C | �ٵμ�ϡHCl �ڵμ�AgNO3��Һ | ���������� ��ɫ���� | ԭ��Һһ����Cl- |
| D | �ٵμ�KCSN��Һ �ڵμ���ˮ | ���������� ��Һ�ʺ�ɫ | ԭ��Һһ����Fe2+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧ�� | H-H | F-F | H-F | H-Cl | H-1 |
| E��kJ/mol�� | 436.0 | 157 | 568 | 431.8 | 298.7 |
| A�� | �������ȶ��Ļ�ѧ����H-F | |
| B�� | 431.8 kJ/mol��E��H-Br����298.7 kJ/mol | |
| C�� | H2��g����2H��g����H=+436.0 kJ/mol | |
| D�� | H2��g��+F2��g����2HF��g����H=-25kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com