
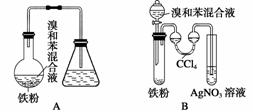
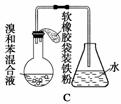
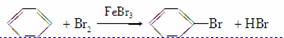
����ͼ��A����ȡ�屽��ʵ��װ�ã�B��C�ǸĽ����װ�á�����ϸ�������Ա�����װ�ã��ش��������⣺
 ��
��
(1)д������װ��������ͬ������������Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
________________________________________________________________________��
д��B���Թ�����������Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________��
(2)װ��A��C�������˳��������ܣ���������
________________________________________________________________________
________________________________________________________________________��
(3)�ڰ�װ��B��Cװ��������ҩƷ��Ҫʹ��Ӧ��ʼ��Ӧ��װ��B���еIJ�����________________________________________________________________________
________________________________________________________________________��
Ӧ��װ��C���еIJ�����________________________________________
________________________________________________________________________��
(4)B�в�����˫��ϴ��������װ�ã���������____________________________
________________________________________________________________________��
��Ӧ��˫��ϴ�����п��ܳ��ֵ�������__________________________��
(5)Bװ�ô����������Ե�ȱ�㣬ʹʵ���Ч�����û����������С�������ȱ����________________________________________________________________________
________________________________________________________________________��
(1)2Fe��3Br2===2FeBr3
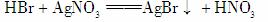
(2)����������
(3)��ת��Һ©���Ļ�����ʹ��ͱ��Ļ��Һ�ε������ϡ���������ʹ����������ͱ���ɵĻ��Һ��
(4)������HBr�ݳ��ı�������Br2��CCl4����ɫ��ɳ�ɫ
(5)��HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵͣ����ڵ��ܲ���AgNO3��Һ�ж��ײ�������
������(1)����FeBr3�����£�����Һ�巢��ȡ����Ӧ����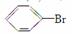 ��ͬʱ����HBr��HBr��AgNO3��Һ����AgBrdz��ɫ������2Fe��3Br2===2FeBr3��
��ͬʱ����HBr��HBr��AgNO3��Һ����AgBrdz��ɫ������2Fe��3Br2===2FeBr3��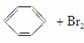
(2)��Ӧ���ȣ��������ӷ�������������л������Ⱦ�����A��Cװ���о������˳��������ܣ���HBr�����������������á�
(3)B����ת��Һ©���Ļ�����ʹ��ͱ��Ļ��Һ�ε������ϣ�C����������ʹ����������ͱ���ɵĻ��Һ�С�
(4)���շ�Ӧ����HBr�ݳ���Br2�ͱ�����������CCl4���ܽ����壬CCl4����ɫ��ɳ�ɫ��
(5)��HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵͣ����ڵ��ܲ���AgNO3��Һ�ж��ײ���������


 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ǰ��ͭ������һ�������仯���� �� ��
A��ͭ˿�ڿ��������պ����������Ҵ� B��ͭƬ�����ữ����������Һ
C��ͭ�����Ļ�������ϡ���� D��ͭ��п��ϡ���ṹ�ɵ�ԭ��طŵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ױ���ú���ۺ����õõ��IJ���֮һ����ṹ��ʽΪ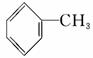 ���Իش��������⣺
���Իش��������⣺
(1)����ױ������ϵΪ________��
A��ͬ���칹�� B��ͬλ��
C��ͬ�������� D��ͬϵ��
(2)�ױ�ȼ��ʱ������Ϊ____________________________________________��
1 mol �ױ���ȫȼ���������������ʵ���Ϊ______________________��
(3)�ױ������ϵ�һ�ȴ�����________�֡�
(4)��֪���� �ṹ�����ʿɱ����Ը��������Һ���������ֱ��ͼױ��ķ�����_____________________________��
�ṹ�����ʿɱ����Ը��������Һ���������ֱ��ͼױ��ķ�����_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڱ������ʵ������У�����ȷ����(����)
A��������ɫ����������ζ��Һ��
B�������±���һ�ֲ�����ˮ���ܶ�С��ˮ��Һ��
C������һ�������������巢��ȡ����Ӧ
D���������е��͵�˫����Ӧ���еķ����ӳɷ�Ӧ�����ԣ��ʲ����ܷ����ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����÷�Һ©�������һ��Һ��������(����)
A����ͱ� B�������屽
C��ˮ�������� D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
50 ��ʱ�����и���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ���� (����)��
A��pH��4�Ĵ����У�c(H��)��4.0 mol��L��1
B������С�մ���Һ�У�c(Na��)��c(HCO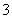 )
)
C������ʳ��ˮ�У�c(Na��)��c(H��)��c(Cl��)��c(OH��)
D��pH��12�Ĵ�����Һ�У�c(OH��)��1.0��10��2 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.100 0 mol��L��1 NaOH��Һ�ζ�20.00 mL 0.100 0 mol��L��1 CH3COOH��Һ���õζ���������ͼ������˵����ȷ���� (����)��
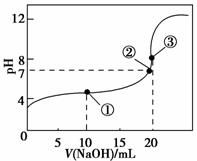
A�������ʾ��Һ�У�c(CH3COO��)��c(OH��)��c(CH3COOH)��c(H��)
B�������ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(CH3COO��)
C�������ʾ��Һ�У�c(Na��)>c(OH��)>c(CH3COO��)>c(H��)
D���ζ������п��ܳ��֣�c(CH3COOH)>c(CH3COO��)>c(H��)>c(Na��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ���ҵ�п��Ƶķ�Ӧ����Ӧ (����)��
A���¶�Խ��Խ��
B��ѹǿԽ��Խ��
C�������������������Խ��Խ��
D����ѡ�Ĵ�������Խ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ�м�������Na2O2�����ܴ���������������ǣ� ��
A��NH4+��Ba2+��Cl����NO3�� B��K+��AlO2����Cl����SO42��
C��Ca2+��Mg2+��NO3����HCO3�� D��Na+��Cl����CO32����SO32��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com