
�Ʊ�Cl2����8 mol•L-1������100mL������12 mol•L-1�����������ơ�
����Ҫ12 mol•L-1����������Ϊ mL����ȷ��0.1 mL��
��Ϊ������ƣ�����������С�ձ��⣬����Ҫѡ�������Ϊ ��
A��100mL��Ͳ B��������ƽ C��100mL����ƿ D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι�
�� ������ƿ��ʹ�÷����У����в����У�����ȷ���� (��д���)��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C�� ������Һʱ����Ͳ��ȡŨ������ò���������������ƿ�У�������������ˮ���ӽ��̶���1cm��2cm�����ý�ͷ�ιܵμ�����ˮֱ����Һ�����ʹ��ͱ�����ƽ
D�����ݺ�Ǻ�ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ�����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡģ���� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
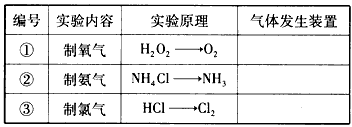
�±���ʵ�����Ʊ�������й����ݣ�
��1�����������У����Ʊ����̿�������ѡ����ʵ�����������ʵ�ֵ��� ��������Ļ�ѧʽ����ͬ�������ӷ�Ӧԭ���������Բ�ͬ����������������� ��
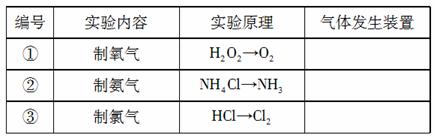
��2�����ݱ�������ʵ��ԭ����������װ����ѡ����ʵ����巢��װ�ã������������ϱ��еĿո��С�[��+��-��*Դ.��]
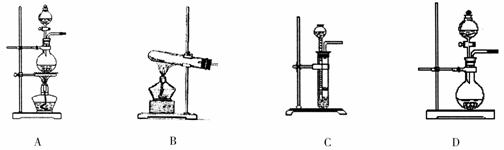
��3�����������Ʊ�O2��װ���Ʊ�NH3����ѡ����Լ�Ϊ ��
��4���Ʊ�Cl2����8 mol��L-1������100mL������12 mol��L-1�����������ơ�
��Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ�����е� ������ţ�
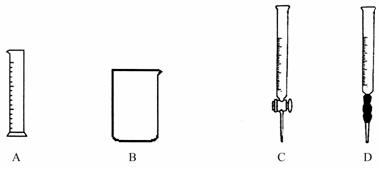
�����ƹ����У�����ͼ���������⣬����Ҫ�������� �� ��
��
�����궨���������Ũ���Ƿ�ȷ�������õ�ʵ�鷽�������� ��
��5�������ſ������ռ�Cl2�������з����ڻ��������ռ�װ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ʊ�Cl2����8 mol•L-1������100mL������12 mol•L-1�����������ơ�
����Ҫ12 mol•L-1����������Ϊ mL����ȷ��0.1 mL��
��Ϊ������ƣ�����������С�ձ��⣬����Ҫѡ�������Ϊ ��
A��100mL��Ͳ B��������ƽ C��100mL����ƿ D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι�
�� ������ƿ��ʹ�÷����У����в����У�����ȷ���� (��д���)��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C�� ������Һʱ����Ͳ��ȡŨ������ò���������������ƿ�У�������������ˮ���ӽ��̶���1cm��2cm�����ý�ͷ�ιܵμ�����ˮֱ����Һ�����ʹ��ͱ�����ƽ
D�����ݺ�Ǻ�ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com